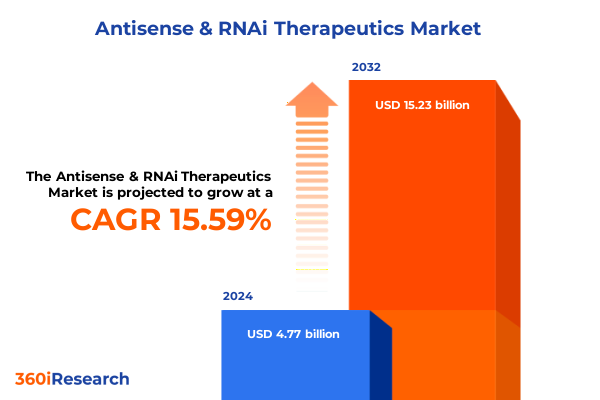
The Antisense & RNAi Therapeutics Market size was estimated at USD 5.46 billion in 2025 and expected to reach USD 6.25 billion in 2026, at a CAGR of 15.76% to reach USD 15.23 billion by 2032.
Strategic Insights into Establishing the Critical Foundation for Antisense and RNAi Therapeutics Evolution Against a Backdrop of Scientific Innovation and Regulatory Advancements
The convergence of molecular biology breakthroughs and precision medicine imperatives has propelled antisense oligonucleotides and RNA interference (RNAi) modalities into the vanguard of therapeutic innovation. Early approvals of antisense therapies laid the groundwork, while the entry of RNAi candidates into late-stage clinical development has fueled a wave of strategic partnerships and high-value licensing agreements. Regulatory agencies globally have adapted frameworks to expedite the approval pathways for oligonucleotide drugs targeting rare and genetic diseases, creating a fertile environment for continued investment and pipeline expansion.
Against this backdrop, the research community is witnessing accelerated evolution in oligonucleotide chemistries, encompassing phosphorothioate backbones and novel sugar modifications designed to enhance target specificity and in vivo stability. Advancements in predictive analytics, coupled with dynamic delivery systems ranging from lipid nanoparticle platforms to GalNAc conjugates, are reshaping the pharmacokinetic and biodistribution profiles of these modalities. As a result, the sector is transitioning from proof-of-concept trials to broad clinical validation across diverse therapeutic areas, positioning antisense and RNAi drugs to address previously intractable disease targets.
This report serves as a foundational compendium for stakeholders seeking to navigate the complex interplay of scientific innovation, regulatory evolution, and commercial dynamics within oligonucleotide therapeutics. It synthesizes emerging trends, delineates critical inflection points in technology adoption, and frames the strategic considerations essential for organizations aiming to capitalize on the next wave of therapeutic breakthroughs.
Charting Innovative Paradigm Shifts Reshaping the Antisense and RNAi Therapeutic Landscape Through Cutting-Edge Technological Breakthroughs, Strategic Partnerships, and Collaborative Ecosystem Models
The landscape of antisense and RNAi therapeutics has undergone a profound transformation as interdisciplinary research converges with biopharma’s strategic priorities. Cutting-edge delivery platforms that were once confined to academic proof-of-concept studies have matured into robust industrial processes, enabling more predictable manufacturing outcomes and scalable production. In parallel, the proliferation of artificial intelligence and machine learning tools has optimized oligonucleotide design, accelerating target validation cycles and reducing attrition rates in early-stage development.
Moreover, ecosystem models are evolving beyond traditional licensing arrangements toward more integrated collaborations. Pharmaceutical incumbents and agile biotech innovators are forging co-development alliances that leverage complementary expertise in chemistry, clinical development, and regulatory strategy. These dynamic partnerships are complemented by increasingly sophisticated engagement with contract research organizations that offer turnkey capabilities spanning in vitro screening through to clinical trial execution.
Transitioning from monolithic research frameworks, the sector now embraces modular innovation, where platform technologies can be readily repurposed for distinct targets. This shift not only amplifies the scope of addressable diseases but also underpins more efficient capital allocation across diverse candidate portfolios. As stakeholders adapt to this new paradigm, strategic differentiation will hinge on delivering value through end-to-end integration, from rational oligonucleotide design to precision targeting in vivo.
Assessing the Far-Reaching and Complex Consequences of 2025 United States Tariff Policies on Supply Chains, Research Costs, Global Competitive Dynamics, and Innovation Trajectories
The implementation of new tariff measures in the United States during 2025 has introduced significant complexity to supply chain management and cost structures within the oligonucleotide therapeutics space. Raw materials essential for synthesis of antisense oligonucleotides and RNAi constructs-ranging from specialty phosphoramidites to lipid nanoparticles-have been subjected to increased import duties. This shift has prompted many manufacturers to reassess sourcing strategies and seek alternative suppliers or domestic production partners to mitigate exposure to sustained cost pressures.
Consequently, research organizations have reported extended lead times and higher procurement expenses, particularly for reagents procured from major export hubs. As supply costs have escalated, companies are intensifying collaboration with contract research organizations that offer vertically integrated services, encompassing both raw material production and downstream formulation expertise. While these strategic realignments help absorb tariff-driven inflation, they can also constrain the flexibility of smaller research entities with limited negotiating leverage.
Furthermore, the cumulative effect of tariff policies has underscored the importance of geographic diversification in manufacturing. Biopharmaceutical companies are increasingly exploring near-shore production facilities and forging alliances with regional providers in the Americas to shore up supply reliability. At the same time, free trade agreements and regulatory reciprocity frameworks are being leveraged to alleviate cross-border barriers. As these dynamics unfold, organizations that proactively recalibrate sourcing and distribution networks will maintain competitive advantage while safeguarding innovation trajectories.
Deriving Actionable Insights from a Multifaceted Segmentation Analysis to Guide Strategic Development Across Oligonucleotide Types, Administration Modes, Therapeutic Areas, and End-User Dynamics
A multifaceted segmentation assessment reveals nuanced opportunities across oligonucleotide type, mode of administration, therapeutic focus, and end-user engagement. Within the oligonucleotide type segment, interfering RNAi submodalities-specifically small interfering RNA and micro interfering RNA constructs-are demonstrating robust pipeline progression, particularly in metabolic and hepatic disease indications. Concurrently, antisense oligonucleotides continue to deliver compelling proofs of concept in neurological and rare disease applications, where precise exon skipping and splice modulation capabilities address high-unmet-need patient populations.
Evaluation of administration modalities indicates that intravenous and subcutaneous delivery routes remain dominant due to established clinical safety profiles and systemic biodistribution advantages. Intrathecal administration retains a critical niche for central nervous system targets, underpinning therapies for spinal muscular atrophy and other genetic neurological disorders. Meanwhile, oral and topical formulations are emerging as promising avenues to enhance patient convenience and expand indications, albeit requiring further innovation to overcome barriers related to mucosal absorption and stability.
Therapeutic segmentation accentuates neurology and rare diseases as high-growth frontiers, driven by favorable regulatory incentives and clear clinical endpoints. Oncology and immunology sectors are also experiencing renewed attention, with next-generation oligonucleotide constructs engineered for tumor-specific gene silencing and immune modulation. Analysis of end-user engagement underscores that pharmaceutical companies are intensifying in-house capabilities, whereas contract research organizations differentiate through specialized service offerings. Research laboratories, with agile academic environments, continue to incubate early-stage targets, feeding the broader ecosystem with novel therapeutic hypotheses.
This comprehensive research report categorizes the Antisense & RNAi Therapeutics market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Oligonucleotide Type
- Mode Of Administration
- Therapeutic Areas
- End User
Revealing Strategic Regional Disparities and Growth Drivers Across the Americas, Europe, Middle East & Africa, and Asia-Pacific Markets in Oligonucleotide Therapeutics
Regional evaluation across the Americas, Europe, Middle East and Africa, and Asia-Pacific regions highlights distinct competitive advantages and emergent challenges. In the Americas, the United States maintains a preeminent position, buoyed by extensive venture capital funding, a mature regulatory framework, and robust domestic manufacturing infrastructure. Canadian research hubs contribute early-stage discovery capabilities, while collaborations across North-South corridors facilitate efficient material flow and clinical trial recruitment.
In Europe, Middle East and Africa, regulatory harmonization within the European Union accelerates market entry, supported by centralized approval processes and orphan drug incentives. The Middle East is witnessing rising investment in biotechnology clusters, aiming to reduce dependence on imported therapeutics. Africa’s landscape is shaped by growing public-private partnerships focused on capacity building and technology transfer, laying the groundwork for future pipeline development.
The Asia-Pacific region stands out for its rapid expansion, driven by government-backed initiatives in China, Japan, and South Korea to scale biomanufacturing capabilities. Cost-effective production and a vast patient pool for clinical trials attract global pharmaceutical companies seeking to diversify development pipelines. Australia and Southeast Asian nations are developing expertise in regulatory science and advanced analytics, fostering an environment where local biotech innovators can engage on a global stage.
This comprehensive research report examines key regions that drive the evolution of the Antisense & RNAi Therapeutics market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Competitive Strategies and Innovation Profiles of Leading Biopharmaceutical Companies Driving Antisense and RNAi Therapeutic Advances, Pipeline Development, and Strategic Collaborations Globally
A review of leading biopharmaceutical players reveals divergent strategies in advancing antisense and RNAi therapeutics. Ionis Pharmaceuticals continues to pioneer antisense modalities with a diverse pipeline targeting neurological disorders, cardiovascular conditions, and rare genetic diseases. Its iterative chemistry optimizations and deep experience navigating regulatory landscapes have positioned the company as a benchmark for antisense development.
Alnylam Pharmaceuticals has asserted leadership within the RNAi domain, leveraging a suite of approved products and strategic partnerships to extend its platform into new indications. Collaborative alliances with global pharmaceutical firms have facilitated commercialization of key assets, while internal R&D efforts persist in refining delivery technologies for extrahepatic targets. Similarly, Arrowhead Pharmaceuticals focuses on next-generation targeted RNAi delivery, exploring ligand-conjugate approaches that promise enhanced tissue specificity.
Novartis, through its acquisition of RNAi technology pioneers and co-development of landmark products, exemplifies how large pharmaceutical organizations integrate oligonucleotide platforms into broader therapeutic portfolios. Silence Therapeutics, with its lipid nanoparticle expertise, and Roche, with a network of research collaborations in gene silencing, further demonstrate the competitive breadth of platform innovators. Emerging companies and academic spin-outs complement this landscape by targeting niche indications and exploring novel chemistries, underscoring an ecosystem rich with collaboration potential.
This comprehensive research report delivers an in-depth overview of the principal market players in the Antisense & RNAi Therapeutics market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Acuitas Therapeutics Inc.
- Alloy Therapeutics, Inc.
- Alnylam Pharmaceuticals, Inc.
- Arbutus Biopharma Corporation
- Arrowhead Pharmaceuticals, Inc.
- AstraZeneca plc
- Bayer AG
- Benitec Biopharma Limited
- City Therapeutics
- Danaher Corporation
- Eli Lilly and Company
- Evox Therapeutics Ltd
- GenScript Biotech Corporation
- GSK PLC
- Ionis Pharmaceuticals, Inc.
- Merck KGaA
- Novo Nordisk A/S
- Pfizer Inc.
- Ribocure Pharmaceuticals AB
- Sanofi S.A.
- Shanghai Argo Biopharmaceutical Co., Ltd.
- Silence Therapeutics plc
- Stoke Therapeutics, Inc.
- Suzhou Ribo Life Science Co., Ltd.
- Wave Life Sciences Ltd
Formulating Targeted and Impactful Strategic Recommendations to Empower Industry Leaders Navigating and Future-Proofing the Complex Antisense and RNAi Therapeutics Ecosystem
To thrive in the evolving oligonucleotide therapeutics landscape, industry leaders should prioritize investment in advanced delivery technologies that enhance target engagement and minimize off-target effects. Strategic partnerships with contract research organizations specializing in vertically integrated services can mitigate supply chain risks and accelerate clinical development timelines. Adopting a diversified sourcing strategy for critical raw materials and leveraging near-shore manufacturing facilities will be essential to maintain resilience against tariff-induced cost fluctuations.
Furthermore, organizations should cultivate regulatory agility by engaging early with health authorities to shape development pathways and capitalize on expedited review programs. Embracing real-world evidence generation and digital health tools can support more robust safety and efficacy datasets, thereby facilitating reimbursement discussions and patient access strategies. In therapeutic areas marked by high unmet need, such as rare genetic disorders, dedicating resources to precision delivery mechanisms and biomarker-driven clinical trial design will yield differentiated value propositions.
Finally, expanding geographic footprints through regional collaborations and technology transfer initiatives will unlock new patient populations while mitigating the impact of localized policy changes. Integrating data analytics and machine learning into candidate selection processes can further refine pipeline prioritization, ensuring that organizational resources are aligned with the most promising targets and commercial landscapes.
Outlining a Rigorous, Transparent, and Comprehensive Research Methodology Featuring Robust Data Collection and Analytical Frameworks to Illuminate Antisense and RNAi Therapeutic Market Dynamics
This report applies a robust and comprehensive research methodology designed to deliver granular insights into the antisense and RNAi therapeutics market. The approach integrates secondary research from peer-reviewed journals, patent databases, clinical trial registries, and regulatory agency announcements to map the current state of technology and approval trajectories. Concurrently, primary research activities include in-depth interviews with senior executives, R&D researchers, and regulatory specialists to validate trends and identify emerging challenges.
A detailed analytical framework underpins the evaluation of segmentation variables, encompassing oligonucleotide chemistries, administration routes, therapeutic areas, and end-user categories. Qualitative assessments of company strategies are supplemented by quantitative analysis of patent filings, collaboration networks, and clinical trial initiations. Regional dynamics are explored through localized stakeholder consultations and examination of government policy documents, ensuring that insights reflect the heterogeneity of global markets.
Throughout the research process, methodological rigor is maintained via data triangulation and iterative validation with subject-matter experts. This layered approach ensures that the findings are both comprehensive and actionable, providing a credible foundation for strategic decision-making across the oligonucleotide therapeutics value chain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Antisense & RNAi Therapeutics market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Antisense & RNAi Therapeutics Market, by Oligonucleotide Type
- Antisense & RNAi Therapeutics Market, by Mode Of Administration
- Antisense & RNAi Therapeutics Market, by Therapeutic Areas
- Antisense & RNAi Therapeutics Market, by End User
- Antisense & RNAi Therapeutics Market, by Region
- Antisense & RNAi Therapeutics Market, by Group
- Antisense & RNAi Therapeutics Market, by Country
- United States Antisense & RNAi Therapeutics Market
- China Antisense & RNAi Therapeutics Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 954 ]
Synthesizing Core Findings and Strategic Implications to Provide a Conclusive, Holistic Overview Guiding Future Developments in Antisense and RNAi Therapeutics
By synthesizing the latest scientific advancements, regulatory evolutions, and commercial strategies, this executive summary delivers a cohesive strategic overview for stakeholders in the antisense and RNAi therapeutics domain. The transformative shifts in delivery technologies, the nuanced impact of tariff policies, and the multi-dimensional segmentation insights collectively underscore a market on the cusp of expansive growth and diversification.
Regional analyses reveal distinct competitive advantages, with established markets in the Americas complemented by dynamic growth corridors across EMEA and Asia-Pacific. The competitive profiles of leading biopharmaceutical players illustrate the ongoing convergence of platform innovation, strategic partnerships, and integrated development models. Against this complex backdrop, the actionable recommendations provided herein offer a clear roadmap for navigating supply chain volatility, optimizing R&D investment, and capitalizing on emergent therapeutic opportunities.
This conclusion affirms that success in the evolving oligonucleotide space will belong to organizations that combine scientific rigor with strategic foresight, while continuously adapting to policy changes and technological breakthroughs. Armed with these insights, decision-makers are better positioned to drive impactful research, secure regulatory endorsements, and deliver novel treatments to patients worldwide.
Empowering Decision-Makers to Access In-Depth Antisense and RNAi Therapeutic Market Research by Connecting with Ketan Rohom for Comprehensive Analysis
For in-depth strategic insights, groundbreaking data, and a comprehensive market landscape assessment of the global antisense and RNAi therapeutics sector, we invite you to connect with Ketan Rohom, Associate Director of Sales & Marketing. Engaging with Ketan will grant your organization direct access to the full research deliverable, empowering your teams with actionable intelligence, tailored executive summaries, and focused deep dives that address your unique strategic priorities. Reach out today to secure your copy of the definitive market research report and position your company at the forefront of innovation in oligonucleotide therapeutics.

- How big is the Antisense & RNAi Therapeutics Market?
- What is the Antisense & RNAi Therapeutics Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




