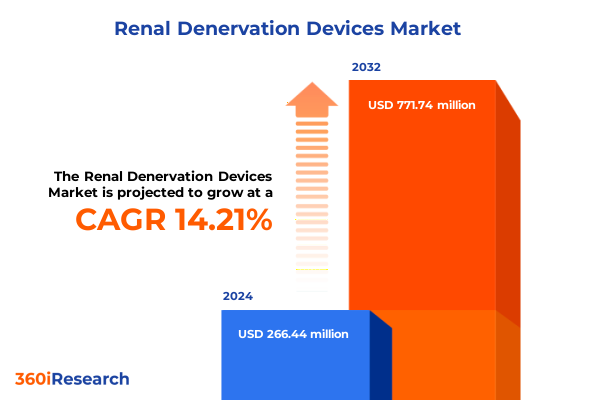
The Renal Denervation Devices Market size was estimated at USD 301.42 million in 2025 and expected to reach USD 342.11 million in 2026, at a CAGR of 14.37% to reach USD 771.74 million by 2032.
Pioneering Renal Denervation Therapeutic Landscape Redefines Resistant Hypertension Management with Innovative Catheter Technologies Driving Clinical Transformations
Renal denervation has emerged as an innovative therapeutic approach for patients facing treatment-resistant hypertension, leveraging catheter-based technology to target the sympathetic nerves that contribute to chronically elevated blood pressure. The U.S. Food and Drug Administration granted approval for renal denervation devices in November 2023, marking a significant milestone in interventional cardiology and offering a viable adjunct to pharmacotherapy for individuals whose blood pressure remains uncontrolled despite multiple medications.
By delivering energy directly to the renal artery wall, renal denervation disrupts aberrant sympathetic signaling pathways, leading to sustained reductions in blood pressure and a potential decrease in cardiovascular event risk. This minimally invasive procedure is executed via percutaneous catheter access, allowing for precise energy delivery through radiofrequency, ultrasound, or chemical ablation techniques.
Early clinical evidence, including data from the SPYRAL-HTN Global Clinical Program, has demonstrated durable blood pressure reductions of up to 18 mmHg over three years, showcasing the potential of renal denervation to offer long-term benefits beyond medication adherence challenges. As a result, leading healthcare institutions are actively evaluating patient eligibility criteria, refining procedural workflows, and engaging with payers to establish sustainable reimbursement pathways.
Against this backdrop of clinical promise and regulatory support, the renal denervation devices space stands at the intersection of advanced catheter technology, evolving patient care models, and shifting reimbursement landscapes. Understanding these elements in concert lays the foundation for strategic positioning and informed investment into the growth trajectory of this novel therapeutic modality.
Emerging Technological Innovations and Care Pathway Evolutions Are Reshaping the Future of Renal Denervation Procedures Worldwide
A convergence of technological advancements is fundamentally reshaping the renal denervation landscape, heralding a new era of multifunctional energy delivery systems. Dual-mode platforms that integrate ultrasound and radiofrequency capabilities are poised to enhance procedural accuracy, reducing treatment variability and optimizing lesion formation at the perirenal nerve interface.
In parallel, artificial intelligence and machine learning algorithms are being embedded into renal denervation systems to support real-time procedural guidance and personalize energy delivery parameters based on patient-specific anatomical and physiological data. This digital infusion aims to elevate procedural consistency and improve clinical outcomes by tailoring ablation patterns to individual nerve distributions.
The outpatient setting is also witnessing accelerated adoption, as streamlined device designs and minimally invasive workflows make same-day discharge a practical reality. Transitioning renal denervation from traditional inpatient environments to ambulatory surgical centers not only drives procedural efficiency but also addresses patient preferences for reduced hospital stays and overall care costs.
A growing emphasis on combination therapies-integrating renal denervation with pharmacological agents targeting renin–angiotensin–aldosterone pathways-is emerging as an innovative strategy to achieve synergistic blood pressure control. Collaborative efforts between device manufacturers and pharmaceutical entities are expected to yield integrated care protocols that leverage the strengths of interventional and medical management for optimal patient outcomes.
Evolving United States Trade Policies and Tariff Adjustments in 2025 Influence Supply Chains and Cost Structures for Renal Denervation Devices
In May 2025, the Office of the United States Trade Representative announced the extension of certain product exclusions from China Section 301 tariffs through August 31, 2025, preserving duty relief for intermediate components and medical-device inputs critical to catheter manufacturing. This extension offers temporary stability for supply chains that depend on China-origin substrates and subassemblies.
Concurrently, the USTR’s final modifications to Section 301 tariffs have recalibrated duties on specific medical products, with syringes and needles facing a 100 percent tariff increase effective September 27, 2024, while surgical and non-surgical respirators will move from 0–7.5 percent to 25 percent in 2024 and further to 50 percent in 2026. Rubber medical gloves are slated to see a jump from 7.5 percent to 50 percent on January 1, 2025, rising again to 100 percent in 2026.
An accompanying notice in June 2025 reiterated that no new product exclusions were introduced, underscoring the importance of actively managing customs classifications and maintaining visibility on tariff exclusion expiration timelines to mitigate unanticipated cost escalations.
Given the reliance on disposable supplies and ancillary tools in renal denervation procedures, this evolving tariff environment demands proactive supply chain strategies. Manufacturers and distributors must assess alternative sourcing options, engage with customs experts to optimize HTSUS classifications, and consider inventory positioning to limit exposure to escalating duties and exclusion sunset events.
Comprehensive Insights into Product Modalities Energy Sources Clinical Applications End Users Distribution Channels and Procedure Types Driving Market Differentiation
The renal denervation device market can be nuanced through multiple dimensions that reveal distinct avenues for product differentiation and customer targeting. Products are categorized by ablation modality-chemical approaches using ethanol alongside radiofrequency systems that offer monopolar or multipolar configurations, and ultrasound platforms available in endovascular or external form factors-each catering to unique clinical preferences and anatomical considerations.
Energy source remains a fundamental market axis, with chemical, radiofrequency, and ultrasound technologies competing across efficacy, procedural efficiency, and safety profiles. Clinicians weigh the ability to deliver precise thermal or neurolytic energy against the potential for vascular trauma or collateral tissue impact when selecting a platform.
Clinical applications further stratify market opportunity, encompassing chronic kidney disease patients in early (Stage 1–2) or advanced (Stage 3–5) stages, individuals with heart failure characterized by preserved or reduced ejection fraction subtypes, and patients with primary or secondary hypertension. This granularity informs clinical trial design and payer coverage criteria, as evidence requirements may vary by patient subgroup.
Finally, adoption is influenced by end user profiles-ranging from ambulatory surgical centers and specialized cardiac institutes to outpatient clinics and full-service hospitals-along with distribution channels that include direct sales and distributor networks, and procedural choices between bilateral or unilateral ablation. Together, these segmentation layers shape commercialization strategies and enable targeted engagement with key stakeholders.
This comprehensive research report categorizes the Renal Denervation Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Energy Source
- Procedure
- Application
- End User
- Distribution Channel
Distinct Regional Dynamics Across Americas EMEA and Asia-Pacific Highlight Varied Adoption Landscapes and Growth Drivers for Renal Denervation Devices
Across the Americas, the United States has emerged as the primary driver of regional adoption, fueled by recent FDA approvals, favorable Medicare transitional pass-through payments, and the initiation of a national coverage analysis that signals broader intentions to integrate renal denervation into standard therapeutic guidelines. Canada and Latin American markets display increasing interest, driven by rising hypertension prevalence and expanding healthcare infrastructure.
In Europe, Middle East & Africa, regulatory processes such as CE marking and country-specific reimbursement negotiations have established Europe as a leading adopter, with nations like Germany and the United Kingdom pioneering coverage frameworks. These markets benefit from unified health technology assessment pathways, though variations in national healthcare funding models continue to influence device uptake and procedural volumes.
The Asia-Pacific region presents a heterogeneous landscape, where advanced markets such as Japan and Australia exhibit early adoption supported by well-developed interventional cardiology centers, while emerging economies including China and India offer substantial growth potential driven by escalating cardiovascular disease burdens and expanding hospital networks. Key challenges center on navigating diverse regulatory environments and establishing robust local evidence generation to support payer value propositions.
Transitioning from established Western markets into Asia-Pacific requires adaptive strategies that account for variable healthcare financing models, clinician training infrastructure, and cultural perceptions of interventional treatments. Success in this region often hinges on local partnerships, targeted clinical education programs, and strategic alignment with national disease management priorities.
This comprehensive research report examines key regions that drive the evolution of the Renal Denervation Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Strategic Competitive Landscape Featuring Medtronic ReCor Medical Abbott and Emerging Innovators Steering Renal Denervation Technology Advancements
Medtronic has secured a transitional pass-through payment designation for its Symplicity Spyral renal denervation catheter effective January 1, 2025, for a three-year period, underscoring payer confidence in the technology’s potential to reduce hypertension-related costs and improving patient access by mitigating upfront hospital expenses.
Following this, Medtronic engaged CMS to initiate a national coverage analysis on January 13, 2025, marking a first-of-its-kind evaluation for an interventional hypertension treatment and signaling a pivotal step toward standardized Medicare coverage, with an expected determination by October 11, 2025.
ReCor Medical’s Paradise ultrasound renal denervation system has demonstrated robust clinical efficacy in the RADIANCE-HTN SOLO trial, where endovascular ultrasound achieved significant systolic and diastolic blood pressure reductions at two months compared to sham controls. This evidence supports the system’s appeal among interventionalists seeking a non-contact ablation mechanism that promises circumferential nerve disruption with minimal operator variability.
Abbott (formerly St. Jude Medical) has established a strong clinical foundation with its EnligHTN multi-electrode radiofrequency catheter, proven safe and effective in early-stage trials that reported sustained office blood pressure reductions of up to 28 mmHg within six months and favorable safety profiles without serious vascular complications. Emerging competitors and collaborations continue to expand the field, introducing novel platforms and strategic partnerships designed to enhance procedural efficiency and broaden patient access.
This comprehensive research report delivers an in-depth overview of the principal market players in the Renal Denervation Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Ablative Solutions, Inc.
- AngioDynamics, Inc.
- Biosense Webster, Inc.
- Boston Scientific Corporation
- Cardionovum GmbH
- Cordis Corporation
- DeepQure Inc.
- Johnson & Johnson Services, Inc.
- Medinol Ltd.
- Medtronic plc
- Mercator MedSystems, Inc.
- Miracor Medical SA
- Otsuka Medical Devices Co., Ltd.
- ReCor Medical, Inc.
- Renal Dynamics Ltd.
- Shanghai Golden Leaf MedTec Co., Ltd.
- Shanghai Wisegain Medical Devices Co., Ltd.
- SoniVie Ltd.
- SoundPipe Therapeutics LLC
- Suzhou SyMap Medical Devices Co., Ltd.
- Symap Medical Co., Ltd.
- Symple Surgical, Inc.
- Terumo Corporation
Actionable Strategies to Navigate Regulatory Reimbursement Partnerships and Innovation Pathways for Sustained Leadership in Renal Denervation
Industry leaders should proactively engage with payers and regulatory agencies to align evidence generation with coverage requirements, ensuring that clinical trial endpoints resonate with both hypertension specialists and reimbursement decision-makers. By fostering early collaboration, manufacturers can streamline the pathway to reimbursement and reduce the time from approval to broad clinical adoption.
Investing in outcome-driven research partnerships and registries will be critical for demonstrating long-term benefits beyond blood pressure reduction, including reductions in cardiovascular events and healthcare utilization. Such real-world evidence can support differentiated value propositions and strengthen negotiations with heterogeneous global payers.
Diversifying technological portfolios through partnerships, licensing agreements, or targeted acquisitions can mitigate single-platform risk while catering to varying physician preferences across energy modalities. Incorporating advanced analytics, remote monitoring capabilities, and procedural support tools will further enhance product differentiation and user adoption.
To navigate the evolving tariff landscape, it is imperative to develop resilient supply chain models that include alternative sourcing options, strategic inventory positioning, and proactive customs classification management. This approach will help buffer against duty fluctuations and maintain cost competitiveness.
Robust Research Framework Integrating Primary Expert Interviews Secondary Data Triangulation and Rigorous Quality Controls Ensures Comprehensive Market Insights
This report leverages a dual-phase research methodology beginning with comprehensive secondary research, which encompasses a review of regulatory filings, clinical trial registries, government tariff notices, company press releases, and peer-reviewed literature to assemble foundational insights and identify data gaps.
Primary research follows, involving structured interviews with key opinion leaders, interventional cardiologists, hospital procurement specialists, and regulatory experts to validate secondary findings and surface real-world perspectives on device adoption, procedural workflows, and payer interactions.
All collected data undergo rigorous triangulation against multiple independent sources, and quality control measures such as consistency checks, source validation, and editorial reviews ensure the accuracy, reliability, and relevance of the analysis presented in this report.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Renal Denervation Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Renal Denervation Devices Market, by Product Type
- Renal Denervation Devices Market, by Energy Source
- Renal Denervation Devices Market, by Procedure
- Renal Denervation Devices Market, by Application
- Renal Denervation Devices Market, by End User
- Renal Denervation Devices Market, by Distribution Channel
- Renal Denervation Devices Market, by Region
- Renal Denervation Devices Market, by Group
- Renal Denervation Devices Market, by Country
- United States Renal Denervation Devices Market
- China Renal Denervation Devices Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1431 ]
Synthesis of Critical Market Dynamics Underscores the Transformative Potential and Strategic Imperatives Driving the Renal Denervation Sector Forward
As renal denervation transitions from investigational therapy to mainstream treatment, its capacity to address uncontrolled hypertension represents both a clinical breakthrough and a commercial opportunity. The confluence of multifaceted energy delivery systems, deepening clinical evidence, and evolving reimbursement policies underscores a market poised for sustainable growth.
Manufacturers and stakeholders must navigate a complex interplay of technological differentiation, regulatory milestones, and trade policy shifts, balancing innovation with operational resilience. Success will rest on the ability to cultivate strategic partnerships, leverage real-world data, and maintain agile supply chain strategies in the face of tariff volatility.
By aligning product development with payer value frameworks and clinician workflow preferences, industry participants can accelerate market adoption and establish renal denervation as a standard-of-care adjunct for resistant hypertension. The insights distilled in this executive summary provide a roadmap for informed decision-making and targeted investment throughout the evolving renal denervation landscape.
Engage Directly with Ketan Rohom to Secure Tailored Renal Denervation Market Intelligence and Drive Strategic Growth Initiatives
If you are seeking unparalleled depth and rigor in understanding the dynamic renal denervation device landscape, reach out to Ketan Rohom, Associate Director, Sales & Marketing. His expertise in guiding strategic research acquisitions and his knowledge of the most critical clinical, technological, and policy developments can help tailor a report that answers your specific questions. By engaging with a dedicated professional focused on delivering insights that empower high-stakes decision-making, you will gain the clarity and confidence needed to navigate this transformative market.

- How big is the Renal Denervation Devices Market?
- What is the Renal Denervation Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




