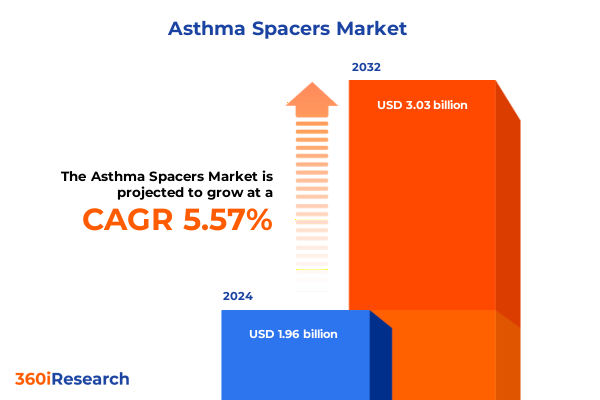
The Asthma Spacers Market size was estimated at USD 2.07 billion in 2025 and expected to reach USD 2.18 billion in 2026, at a CAGR of 5.60% to reach USD 3.03 billion by 2032.
Navigating the Rapidly Evolving Landscape of Asthma Spacer Technologies and Patient-Centered Respiratory Care Solutions During a Period of Unprecedented Healthcare Innovation
Asthma remains a major public health challenge in the United States, with recent surveillance data indicating that approximately 26.8 million Americans-8.2% of the population-had current asthma in 2022, including 4.5 million children under age 18 and 22.3 million adults over 18. The chronic nature of asthma, characterized by airway inflammation and bronchoconstriction, necessitates effective delivery of inhaled medications to control symptoms and prevent exacerbations. Metered-dose inhalers (MDIs) supplemented with spacer devices play a critical role in ensuring optimal drug deposition in the lower airways, reducing oropharyngeal deposition, and compensating for poor hand-breath coordination.
How Technological Advancements and Changing Patient Preferences Are Redefining the Asthma Spacer Industry with Digital Health and Sustainable Materials
The asthma spacer market is undergoing a profound transformation driven by the integration of digital health technologies. Gamified mobile platforms and digital therapeutics now pair with smart devices to boost patient engagement and self-management, as exemplified by recent launches of evidence-backed asthma management apps with features like real-time symptom tracking, air quality alerts, and wearable integration. This convergence of hardware and software is elevating spacer devices from simple add-on chambers to intelligent endpoints within a broader connected care ecosystem.
Simultaneously, telehealth and remote patient monitoring solutions are redefining how clinicians and patients interact. Advanced spacer systems equipped with Bluetooth-enabled sensors and mobile spirometry capabilities enable continuous monitoring of inhalation technique and lung function, facilitating proactive treatment adjustments and reducing avoidable exacerbations. This shift reflects a larger trend toward personalized, data-driven respiratory care where treatment plans are dynamically tailored to patient-specific patterns and environmental triggers.
Additionally, sustainability considerations are reshaping product design and manufacturing. Rising awareness of environmental impact has spurred the development of eco-friendly spacer materials, including biodegradable polymers and recyclable silicone variants, aligning with global healthcare commitments to reduce waste and carbon footprints. Amid these developments, regulatory agencies are issuing guidelines to support innovative device approvals and streamline market entry of next-generation spacers.
Assessing the Cumulative Impact of Recent U.S. Tariff Policies on Imported Asthma Spacer Components and Medtech Supply Chains Through 2025
In 2025, renewed USTR enforcement of Section 301 tariffs on Class I and II medical devices, including respiratory aids, has significantly impacted supply chain dynamics. These measures target imports of specialized device components from China, introducing an additional 10% duty on top of existing rates and prompting manufacturers to reevaluate sourcing strategies to mitigate cost pressures and supply risks.
The White House’s announcement of new China tariffs in May 2024 extended duties to a range of medical products-levying 50% on syringes and needles and 25% on respirators and face masks-which directly influences the cost base of spacer assembly lines that rely on precision medical-grade materials.
Further increases under revived Trump-era tariffs raised duties on surgical and non-surgical respirators to 25% as of September 27, 2024, and imposed tariff hikes up to 100% on syringes and needles starting January 1, 2025. Rubber medical gloves also saw rates double to 50% in 2025, with a second round to 100% in 2026, reshaping the global trade flows of ancillary disposable components.
Industry responses have been mixed: leading medtech firms project substantial profit impacts, with J&J’s MedTech division estimating a $400 million hit in 2025 from these levy increases, while trade associations lobby aggressively for carve-outs and exemptions to preserve patient access and curb healthcare inflation.
Uncovering Critical Market Segmentation Dimensions That Drive Differentiation and Growth Opportunities in the Asthma Spacer Sector
Market segmentation reveals the diverse drivers of growth and differentiation within the asthma spacer sector. Devices are primarily classified into conventional spacers and valved holding chambers, with valved variants further differentiated into disposable and reusable holding chambers based on design and clinical preference. End users span clinics, home care settings, and hospitals, each demanding tailored performance features, from ease of use for pediatric home administration to rigorous sterilization standards for hospital inventories. Distribution channels range from hospital pharmacies to online pharmacies and retail outlets, reflecting the evolving purchasing patterns of healthcare systems and consumers. Product types include disposable spacers for single-patient use and reusable spacers designed for repeated sterilization and long-term deployment. Materials selection-metal, plastic, and silicone-impacts device durability, patient comfort, and cost, while age groups-adult, geriatric, and pediatric-drive size, interface, and instructional design considerations. By understanding these segmentation dimensions, stakeholders can align product portfolios with specific clinical needs, regulatory requirements, and patient preferences.
This comprehensive research report categorizes the Asthma Spacers market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Device Type
- Product Type
- Material
- Age Group
- End User
- Distribution Channel
Examining Regional Market Dynamics and Growth Drivers Across the Americas, EMEA, and Asia-Pacific in Asthma Spacer Adoption and Access
Regional dynamics in asthma spacer adoption reflect both healthcare infrastructure maturity and local policy environments. In the Americas, high asthma prevalence combined with well-established reimbursement frameworks and widespread digital health adoption has accelerated uptake of smart spacers and telehealth-enabled delivery programs, particularly in the U.S. where national surveillance data inform targeted intervention strategies. Transitioning to Europe, Middle East & Africa, regulatory harmonization via CE marking and increasing prioritization of patient-reported outcomes have fostered a competitive market for advanced valved holding chambers, while emerging EMEA markets present opportunities for cost-effective, reusable spacer solutions to address resource constraints. Asia-Pacific markets exhibit rapid growth driven by rising respiratory disease burden, expanding healthcare access, and local manufacturing initiatives that reduce reliance on imports. Government incentives aimed at building regional medtech capacity are catalyzing partnerships between global device leaders and domestic OEMs, ensuring broader availability of next-generation spacers across diverse patient populations.
This comprehensive research report examines key regions that drive the evolution of the Asthma Spacers market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Exploring the Strategic Moves and Innovations of Leading Industry Players Shaping the Future of Asthma Spacer Solutions Worldwide
AptarGroup stands at the forefront of drug delivery innovation, leveraging its core expertise in metered-dose inhaler valve technology and its strategic investments in digital health. The company’s global manufacturing footprint supports scalable production of precision valves and actuator assemblies, while recent partnerships with digital therapeutics firms have positioned Aptar as a pivotal enabler of connected inhalation solutions.
Trudell Medical International has solidified its leadership in valved holding chamber design, with its AeroChamber brand holding a dominant market position in over 110 countries. Supported by hundreds of peer-reviewed clinical evaluations, Trudell’s devices are recognized for their reliability in enhancing drug delivery and mitigating coordination challenges, earning recommendations from leading pharmaceutical companies and professional societies.
Cipla Limited leverages its strength in respiratory drug formulation and generics manufacturing to offer integrated inhalation therapy solutions, collaborating on digital inhaler platforms and telehealth programs that facilitate adherence monitoring and patient education. With a global footprint spanning 47 manufacturing locations, Cipla’s commitment to cost-effective respiratory care makes it a preferred partner for public health initiatives in emerging markets.
This comprehensive research report delivers an in-depth overview of the principal market players in the Asthma Spacers market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- 3M Health Care
- AGP‑Med
- Air Liquide Healthcare
- AstraZeneca plc
- Bird HealthCare Pty Ltd.
- Cipla Limited
- Clement Clarke International Limited
- Drive DeVilbiss Healthcare
- GlaxoSmithKline plc
- HAAG‑STREIT Group
- Instrumentation Industries Inc.
- Koninklijke Philips N.V.
- Koo Medical Equipment Co., Ltd.
- Laboratoire ProtecSom
- Lupin Limited
- Merck & Co., Inc.
- Monaghan Medical Corporation
- Omron Healthcare Co., Ltd.
- PARI GmbH
- Rossmax International Ltd.
- Smiths Medical ASD, Inc.
- SunMed Holdings LLC
- Teleflex Incorporated
- Trudell Medical International Inc.
- Vyaire Medical, Inc.
Actionable Strategies for Stakeholders to Capitalize on Emerging Trends, Strengthen Market Position, and Enhance Patient Outcomes in Asthma Spacer Deployment
Industry leaders should prioritize end-to-end integration of advanced device capabilities with digital health platforms to drive sustained patient engagement and real-world evidence generation. Establishing cross-functional partnerships with telemonitoring providers and AI analytics firms will enable predictive maintenance of inhalation technique and real-time intervention at the point of care. To mitigate tariff-induced cost pressures, companies must diversify supply chain networks by regionalizing component sourcing and expanding near-shore manufacturing hubs. Embracing circular economy principles through development of recyclable and reusable spacer lines will address both environmental sustainability goals and cost optimization. Collaboration with payers and healthcare systems to demonstrate value-based outcomes will foster favorable reimbursement pathways for premium spacer technologies, while targeted educational programs for clinicians and patients will ensure proper device utilization and adherence.
Detailing a Rigorous Research Framework Combining Primary and Secondary Data Sources to Deliver Comprehensive Insights on the Asthma Spacer Market
This analysis combines comprehensive secondary research with primary data collection to ensure robust market insights. Secondary sources include peer-reviewed journals, government surveillance data, regulatory filings, and industry press releases, ensuring a fact-based foundation. Primary research involved structured interviews with key opinion leaders including pulmonologists, respiratory therapists, procurement executives, and device engineers to validate trends and quantify clinical adoption drivers. Data triangulation was conducted by cross-verifying interview findings with quantitative datasets and third-party trade statistics. Segment and regional analyses were structured using standardized frameworks, while tariff impact assessments were modeled using publicly available USTR schedules and industry financial disclosures. This multi-method approach ensures a balanced, impartial, and nuanced depiction of the asthma spacer market landscape.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Asthma Spacers market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Asthma Spacers Market, by Device Type
- Asthma Spacers Market, by Product Type
- Asthma Spacers Market, by Material
- Asthma Spacers Market, by Age Group
- Asthma Spacers Market, by End User
- Asthma Spacers Market, by Distribution Channel
- Asthma Spacers Market, by Region
- Asthma Spacers Market, by Group
- Asthma Spacers Market, by Country
- United States Asthma Spacers Market
- China Asthma Spacers Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1272 ]
Synthesis of Critical Insights Highlighting Opportunities and Challenges in Improving Respiratory Care Through Advanced Asthma Spacer Solutions
The asthma spacer market is poised for sustained expansion fueled by digital transformation, regulatory support for innovative device approvals, and growing patient demand for personalized respiratory care. While tariff pressures introduce cost and supply chain challenges, they concurrently incentivize localization of critical component manufacturing and enhanced device versatility. Segmentation analysis underscores opportunities across end-user settings, age groups, and distribution channels, guiding stakeholders in portfolio optimization. Regional market dynamics reveal a heterogeneous landscape where developed markets seek premium solutions and emerging markets prioritize cost-effective, scalable device models. Collectively, these insights affirm that strategic alignment of technology, supply chain resilience, and stakeholder collaboration will define success in the rapidly evolving respiratory care ecosystem.
Connect with Ketan Rohom to Secure Your Comprehensive Asthma Spacers Market Research Report and Stay Ahead in Respiratory Care Innovation
We invite you to engage directly with Ketan Rohom, Associate Director of Sales & Marketing, to gain tailored insights and strategic guidance on the evolving asthma spacer landscape and secure your comprehensive market research report without delay.

- How big is the Asthma Spacers Market?
- What is the Asthma Spacers Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




