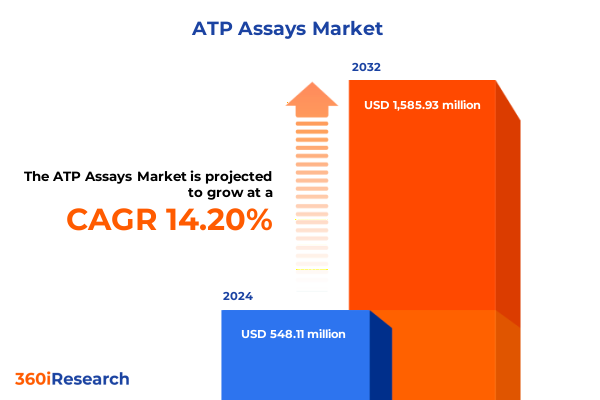
The ATP Assays Market size was estimated at USD 619.65 million in 2025 and expected to reach USD 705.08 million in 2026, at a CAGR of 14.36% to reach USD 1,585.93 million by 2032.
Unveiling the Critical Role of ATP Assays in Modern Life Science Laboratories and Their Rising Importance Across Diverse Industrial Sectors
ATP assays have emerged as indispensable tools for quantifying the presence of adenosine triphosphate in a variety of biological and environmental samples, serving as a fundamental indicator of cellular viability, contamination levels, and microbial activity. Laboratories across academic research institutes and pharmaceutical companies rely on these assays to validate cell culture experiments, monitor bioprocessing operations, and ensure sterility in critical manufacturing environments. In parallel, environmental agencies and food safety teams deploy ATP measurements to rapidly assess hygiene standards and detect potential spoilage or contamination, underscoring the versatility and wide-ranging applicability of this analytical technique.
As technological capabilities have evolved, the demand for rapid, accurate, and cost-effective ATP detection methods has intensified. Innovations in luminometric detection, streamlined assay kits, and portable instrumentation have facilitated near real-time monitoring, enabling stakeholders to make informed decisions more quickly than ever before. This heightened responsiveness has led to improved quality control procedures in hospitals and diagnostic laboratories, where turnaround time is directly linked to patient outcomes. Consequently, ATP assays now occupy a strategic position within multidisciplinary workflows, bridging scientific research and operational efficiency.
Exploring How Technological Innovations and Digital Integration Have Revolutionized ATP Assay Workflows and Accelerated Analytical Precision
Recent years have witnessed a profound transformation in ATP assay workflows, driven by the integration of advanced detection technologies and digital analytics platforms. Traditional manual kits, once reliant solely on colorimetric or fluorescent readouts, have been complemented by automated luminometric systems that offer heightened sensitivity and throughput. These systems seamlessly integrate with laboratory information management software, allowing for streamlined data capture, analysis, and reporting, which in turn accelerates research timelines and enhances reproducibility across experimental protocols.
Moreover, the growing emphasis on point-of-care testing and field-deployable solutions has catalyzed the development of portable luminometers and microfluidic assay platforms. Such innovations support on-site microbial detection for environmental monitoring and food safety inspections, reducing the need for extensive sample transport to centralized facilities. Additionally, the incorporation of artificial intelligence and machine learning algorithms into ATP assay data analysis is opening new frontiers in predictive maintenance and trend forecasting. As a result, research teams and industrial operators alike are empowered to conduct proactive quality assessments and optimize process controls in real time.
Assessing the Holistic Effects of Recent United States Tariff Measures on Supply Chains Cost Structures and Market Dynamics for ATP Assay Solutions
The introduction of targeted tariff measures by the United States in early 2025 has prompted a recalibration of supply chain strategies for ATP assay manufacturers and end users. Instruments and reagents imported from key manufacturing hubs now face elevated import duties, contributing to incremental cost pressures across the procurement cycle. In response, leading vendors have accelerated the localization of production facilities, forging partnerships with domestic suppliers to mitigate the financial impact and safeguard consistent product availability.
In addition, tariff-related customs delays have influenced lead times, compelling laboratories to adjust inventory management practices and adopt buffer stock protocols. Consequently, procurement teams are increasingly collaborating with service providers to implement just-in-time delivery mechanisms and contract testing arrangements that ensure uninterrupted workflow continuity. Furthermore, the cumulative impact of these tariffs has intensified the focus on alternative sourcing regions, encouraging a shift toward diversified logistics networks that span emerging markets in Southeast Asia and Latin America. As organizations continue to navigate these evolving trade dynamics, strategic agility and resilient supply models have become indispensable for maintaining competitive advantage in the ATP assay landscape.
Delving into Comprehensive Market Segmentation Insights Derived from Product Type Technology End User and Sample Type Perspectives
When evaluating the market by product type, instruments comprise both microplate readers and portable luminometers, each addressing distinct throughput and field-deployable use cases. Simultaneously, the kit segment encompasses automated assay formats that integrate seamlessly with robotics platforms, alongside manual kits that deliver flexibility for bespoke experimental protocols. Complementing these offerings, service providers deliver comprehensive contract testing solutions and custom assay development programs, enabling end users to access specialized expertise and reduce internal resource burdens.
From a technology standpoint, luminescence continues to dominate due to its superior sensitivity, with both bacterial luciferase and firefly luciferase variants powering next-generation detection platforms. Colorimetric and fluorescence techniques remain integral for certain applications, offering cost-effective alternatives where ultra-low detection limits are not required. Collectively, these technological modalities cater to diverse analytical requirements, fostering innovation in assay design and performance optimization.
End users span academic research institutes pushing the boundaries of cellular biology, environmental agencies focused on water and soil quality assessments, food and beverage companies ensuring product safety, hospitals and diagnostic laboratories prioritizing rapid contamination checks, and pharmaceutical and biotechnology firms driving drug discovery and bioprocess monitoring. The versatility of ATP assays further extends to sample types that include blood matrices, various food products, soil specimens, swab samples, and water, underscoring the technique’s adaptability across complex sample preparation workflows.
This comprehensive research report categorizes the ATP Assays market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Technology
- Sample Type
- End User
Examining Critical Regional Variations and Growth Drivers Influencing the Adoption of ATP Assay Technologies in the Americas EMEA and Asia Pacific Regions
In the Americas, extensive investment in life science infrastructure and rigorous regulatory frameworks have bolstered the uptake of ATP assay technologies. North American pharmaceutical hubs and Latin American environmental monitoring initiatives benefit from robust distribution channels and established laboratory networks, contributing to streamlined assay deployment across clinical and industrial domains. Meanwhile, in Europe, Middle East, and Africa, stringent hygiene standards and environmental directives have catalyzed demand in food safety and contamination control applications, with regional players emphasizing compliance-driven solutions and sustainable assay formats.
Across the Asia-Pacific region, rapid industrialization and expanding public health initiatives have created a fertile environment for ATP assay adoption. China’s growing biotechnology sector and India’s thriving pharmaceutical landscape have driven substantial investments in modern analytical instrumentation, while Southeast Asian markets leverage portable luminometric platforms for field testing in agricultural and aquaculture settings. Consequently, regional dynamics shaped by economic diversification and technology transfer partnerships continue to redefine competitive positioning and growth trajectories for market participants in this segment.
This comprehensive research report examines key regions that drive the evolution of the ATP Assays market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Uncovering Strategic Moves Competitive Positioning and Innovation Focus Among Leading Vendors Shaping the Future of ATP Assay Solutions in the Market
Leading vendors in the ATP assay space have prioritized strategic partnerships and innovation pipelines to maintain competitive differentiation. Instrument manufacturers have expanded their portfolios through acquisitions of specialized optics and detection technology firms, reinforcing capabilities in high-throughput and portable assay formats. Concurrently, kit developers have invested in reagent formulation advancements, enhancing stability and shelf life for use in challenging field environments.
Service providers, for their part, have scaled contract testing capacities by establishing multi-location laboratories equipped with standardized protocols, thereby reducing turnaround times and increasing flexibility for global clients. Several key companies have also ventured into collaborative research agreements with academic institutions to validate novel assay formats and drive regulatory approvals. By aligning R&D investments with end-user priorities-such as rapid microbial detection in pharmaceutical manufacturing and comprehensive hygiene monitoring in the food sector-these industry leaders are shaping the roadmap for next-generation ATP assay solutions.
This comprehensive research report delivers an in-depth overview of the principal market players in the ATP Assays market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- 3M Company
- AAT Bioquest, Inc.
- Abcam plc
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- BioThema AB
- Biotium, Inc.
- Canvax Biotech S.L.
- Cayman Chemical Company
- Cell Signaling Technology, Inc.
- Danaher Corporation
- Hygiena LLC
- Kikkoman Biochemifa Co., Ltd.
- Lonza Group Ltd.
- Merck KGaA
- Neogen Corporation
- PerkinElmer, Inc.
- Promega Corporation
- Thermo Fisher Scientific Inc.
Formulating Targeted Strategic Recommendations to Enhance Operational Efficiency and Drive Innovation in ATP Assay Development and Deployment Across Industries
Industry leaders can enhance their competitive stance by fostering cross-functional collaboration between R&D, supply chain, and customer success teams to accelerate product iterations and address emerging application demands. Investing in modular instrument platforms that support multiple detection modalities will enable organizations to cater to diverse end-user requirements while optimizing production costs. Furthermore, developing strategic alliances with regional distributors and service networks can facilitate agile market entry and localized support, which is critical in navigating tariff landscapes and regulatory complexities.
Moreover, embedding predictive analytics into ATP assay data workflows can unlock operational efficiencies by preempting supply disruptions and optimizing resource allocation. Companies should also prioritize sustainable reagent formulations and energy-efficient instrumentation, aligning with global ESG objectives and end-user preferences for eco-conscious solutions. By adopting a customer-centric innovation approach and leveraging digital ecosystems for seamless integration, industry stakeholders can capture new growth opportunities and reinforce their leadership in the evolving ATP assay market.
Detailing the Rigorous Research Methodology Employed to Gather Comprehensive Market Data and Ensure Robust Analysis of ATP Assay Industry Trends
The research methodology underpinning this report combined qualitative expert interviews with quantitative data aggregation to ensure comprehensive coverage of the ATP assay market. Primary research engaged key opinion leaders across academic, clinical, and industrial sectors to validate technology trends, end-user pain points, and regional adoption nuances. Simultaneously, secondary sources, including peer-reviewed journals, regulatory publications, and corporate disclosures, were systematically reviewed to corroborate primary insights and contextualize emerging innovations.
Data triangulation was employed to reconcile information from multiple sources, ensuring accuracy and minimizing bias. Vendor profiling relied on a standardized framework evaluating product portfolios, strategic initiatives, and service capabilities. Regional market dynamics were assessed by mapping regulatory environments, infrastructure maturity, and economic indicators against end-user demand patterns. Finally, the report leveraged scenario analysis to examine the potential effects of trade policies and technological disruptions, providing a robust foundation for strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our ATP Assays market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- ATP Assays Market, by Product Type
- ATP Assays Market, by Technology
- ATP Assays Market, by Sample Type
- ATP Assays Market, by End User
- ATP Assays Market, by Region
- ATP Assays Market, by Group
- ATP Assays Market, by Country
- United States ATP Assays Market
- China ATP Assays Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1431 ]
Synthesizing Key Findings and Strategic Implications to Offer a Comprehensive Concluding Perspective on the Evolving ATP Assay Market Dynamics
This analysis synthesizes the critical drivers, challenges, and strategic imperatives shaping the ATP assay landscape. Technological advancements in luminescence detection and digital integration have spurred the development of versatile assay platforms capable of addressing high-throughput laboratory requirements as well as decentralized field testing scenarios. Concurrently, evolving trade policies have underscored the importance of supply chain resilience and localized manufacturing to mitigate cost pressures and delivery uncertainties.
Segmentation insights reveal a diversified market structure, with distinct growth pockets across instrument types, assay formats, end-user applications, and sample categories. Regional variations further highlight opportunities in matured markets characterized by stringent compliance requirements, as well as in emerging economies where investment in life science infrastructure is accelerating. Key players continue to differentiate through innovation pipelines, strategic partnerships, and service expansions, reinforcing a competitive landscape that prioritizes customer-centric solutions and sustainable practices.
Looking ahead, stakeholders who embrace flexible technology architectures, data-driven decision frameworks, and collaborative ecosystem models will be best positioned to capitalize on the evolving dynamics of the ATP assay market. This comprehensive perspective equips decision-makers with the context and foresight needed to navigate shifting market forces and drive long-term value creation.
Take the Next Step in Harnessing Cutting Edge ATP Assay Market Insights by Engaging with Ketan Rohom for a Customized Research Report Purchase
To explore this comprehensive ATP assay market research report and uncover actionable insights tailored to your unique needs, reach out directly to Ketan Rohom, Associate Director, Sales & Marketing, to initiate a personalized consultation session. By engaging with this report, you will gain unparalleled clarity on the latest technological advances, regulatory impacts, segmentation analyses, and regional dynamics shaping ATP assay adoption. Don’t miss the opportunity to leverage expert strategic guidance and secure a competitive advantage in a rapidly evolving landscape. Contact Ketan Rohom today to purchase your copy of the full market research report and empower your organization’s next phase of growth.

- How big is the ATP Assays Market?
- What is the ATP Assays Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




