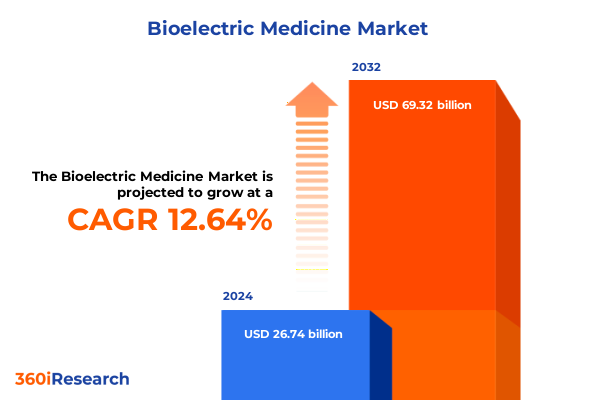
The Bioelectric Medicine Market size was estimated at USD 30.10 billion in 2025 and expected to reach USD 33.88 billion in 2026, at a CAGR of 12.65% to reach USD 69.32 billion by 2032.
Unveiling the Rising Force of Bioelectric Medicine as the Next Frontier in Therapeutic Innovation and Patient-Centric Care for Global Healthcare Transformation
The field of bioelectric medicine is experiencing an unprecedented rise as a transformative therapeutic paradigm that bridges engineering ingenuity with the complexities of human physiology. In recent years, academic breakthroughs and clinical successes have elevated neuromodulation from a niche experimental domain into a mainstream option for addressing a variety of chronic and acute health conditions. This shift owes much to advancements in device miniaturization, improvements in electrode materials, and deeper insights into neural circuit mapping, all of which are converging to deliver precise, patient-centric interventions. Furthermore, the growing emphasis on non-pharmacological therapies has galvanized interest from clinicians, payers, and investors alike, catalyzing funding initiatives that support large-scale clinical trials and regulatory submissions.
As healthcare stakeholders grapple with rising costs and the limitations of systemic drug therapies, bioelectric medicine presents a compelling alternative to modulate physiological processes at their source. The rising prevalence of chronic pain, neurological disorders, and cardiovascular irregularities underscores an urgent need for innovative solutions. Consequently, bioelectric therapies are being integrated into multidisciplinary treatment pathways, supported by data-driven evidence and real-world outcomes. This introduction sets the stage for a detailed examination of how the intersection of cutting-edge technology, shifting policy landscapes, and evolving patient expectations is shaping the future of medical care.
Mapping the Paradigm Shift in Bioelectric Medicine Fueled by Technological Breakthroughs, Regulatory Evolution, and Evolving Patient Expectations
The landscape of bioelectric medicine is undergoing a profound transformation fueled by breakthroughs in both foundational science and applied engineering. Over the past eighteen months, next-generation electrode materials and closed-loop stimulation algorithms have significantly enhanced the safety and efficacy profiles of neuromodulation devices. These innovations are enabling real-time, adaptive therapies that respond to fluctuating patient biomarkers, thereby optimizing therapeutic outcomes while minimizing adverse events. Meanwhile, regulatory bodies worldwide are recalibrating their frameworks to accommodate the unique risk-benefit profiles of implantable and nonimplantable systems, reducing approval timelines for devices demonstrating robust safety data.
Moreover, shifting patient expectations are accelerating demand for minimally invasive, home-based solutions that offer greater convenience and sustained engagement. Digital health integration, including smartphone-paired controls and remote monitoring platforms, is enhancing adherence and providing clinicians with actionable insights into patient progress. As insurers and healthcare networks recognize the long-term cost efficiencies of reduced hospital readmissions and improved functional outcomes, they are increasingly incorporating bioelectric therapies into reimbursement policies. Together, these factors are catalyzing a paradigm shift in how care is delivered and financed, propelling bioelectric medicine toward broader acceptance and adoption within mainstream healthcare ecosystems.
Evaluating the Ripple Effects of 2025 United States Tariff Implementation on Bioelectric Therapeutics Supply Chains, Costs, and Industry Collaboration Dynamics
In 2025, the implementation of targeted United States tariffs on electronic components and specialized biomedical materials has introduced both challenges and opportunities across the bioelectric medicine value chain. Manufacturers of implantable neuromodulation systems and external stimulator devices have faced higher input costs for critical semiconductors, precision microelectrodes, and biocompatible polymers. These tariff-driven cost pressures have prompted device makers to reassess their sourcing strategies, forging closer alliances with domestic suppliers and exploring alternative material formulations without compromising biocompatibility or device longevity.
Simultaneously, rising component costs have triggered a wave of process optimization initiatives aimed at streamlining manufacturing workflows and reducing yield loss. Organizations are investing in advanced automation and lean production methodologies to preserve margins while maintaining quality standards. From a market perspective, the tariffs have also spurred collaborative efforts among industry consortia to advocate for tariff exemptions on high-value biomedical imports, emphasizing the broader public health benefits of accessible neuromodulation therapies. These collective actions are reshaping supply chain resilience and driving strategic innovation in cost management, ultimately influencing how bioelectric devices are priced, manufactured, and distributed throughout the United States.
Unlocking Profound Segmentation Insights in Bioelectric Medicine to Illuminate Targeted Opportunities across Technology, Application, End Users, and Indications
Unlocking profound insights into the bioelectric medicine market requires a multidimensional segmentation approach that reveals targeted opportunities across technology, application, end-user, and indication. When viewed through the lens of technology, the market divides into implantable and nonimplantable neuromodulation systems, with implantables encompassing deep brain stimulation, spinal cord stimulation, and vagus nerve stimulation, and nonimplantables including electric muscle stimulators, iontophoresis devices, and TENS units. This technological breakdown highlights distinct innovation trajectories and regulatory pathways for each category, underscoring the need for specialized R&D roadmaps and tailored clinical trial designs.
From an application standpoint, these therapies are employed across cardiovascular treatment, movement disorder management, neurological rehabilitation, and pain management, each demanding unique performance attributes and patient engagement strategies. Examining end-user segmentation, hospitals and specialty clinics have emerged as early adopters of complex implantable solutions, while ambulatory surgical centers and homecare environments are increasingly favoring portable nonimplantable platforms for chronic pain and rehabilitation. Finally, indication-based segmentation reveals concentrated opportunities within chronic pain relief, epilepsy management, Parkinson’s disease modulation, and post-stroke rehabilitation. Integrating these segmentation dimensions offers a strategic blueprint for aligning product portfolios with evolving clinical needs and optimizing go-to-market approaches at every level of the healthcare ecosystem.
This comprehensive research report categorizes the Bioelectric Medicine market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Technology
- Indication
- Application
- End User
Deciphering Regional Growth Patterns in the Bioelectric Medicine Domain across Americas, Europe Middle East and Africa, and Asia-Pacific Markets
Regional dynamics in bioelectric medicine are shaping the competitive contours of the industry, with each geography exhibiting unique growth drivers and adoption profiles. In the Americas, a mature ecosystem of research institutions and advanced healthcare infrastructure has positioned the region as a leader in clinical trials and early regulatory approvals. Strong reimbursement frameworks in key markets have facilitated widespread access to both implantable and nonimplantable therapies, enabling rapid post-market surveillance and iterative device enhancements.
Across Europe, the Middle East, and Africa, diversity in regulatory regimes and healthcare funding models underscores the importance of adaptive market strategies. Some EMEA nations are accelerating device approvals through streamlined health technology assessment processes, while others emphasize real-world evidence to support reimbursement decisions. Concurrently, emerging economies within the region are witnessing growing demand for cost-effective, portable neuromodulation solutions that address underserved patient populations.
Meanwhile, the Asia-Pacific landscape is characterized by robust investment in manufacturing capabilities and an expanding pool of clinical investigators. Nations such as Japan, South Korea, and Australia are advancing regulatory harmonization efforts, while Southeast Asian markets are demonstrating high growth potential for home-based rehabilitation devices. Together, these regional insights provide a nuanced understanding of where innovation, market access, and patient demand are coalescing to drive the next wave of growth in bioelectric medicine.
This comprehensive research report examines key regions that drive the evolution of the Bioelectric Medicine market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Revealing Strategic Moves of Leading Bioelectric Medicine Companies to Drive Competitive Advantage through Innovation, Partnerships, and Market Diversification
Leading bioelectric medicine companies are leveraging strategic initiatives to deepen their competitive moats and expand global reach. Key players have accelerated their innovation pipelines through collaborations with academic centers, integrating advanced neuromodulation algorithms and biocompatible materials to enhance device performance. At the same time, targeted acquisitions have enabled some organizations to broaden their product portfolios, incorporating complementary technologies such as wearable sensors, digital therapeutics, and AI-driven data analytics platforms.
In parallel, several manufacturers have entered strategic partnerships with contract research organizations and healthcare systems to de-risk clinical development pathways and expedite time to market. These alliances often include shared investment in real-world evidence studies, providing robust outcome data to support both regulatory submissions and payer negotiations. Furthermore, portfolio diversification efforts are underway, with companies exploring adjacent therapy areas such as bioelectric immunomodulation and cardiac rhythm management to leverage existing device platforms and R&D infrastructure.
By harnessing these strategic moves-ranging from joint development agreements to targeted bolt-on acquisitions-companies are positioning themselves to capture emerging opportunities across the full spectrum of bioelectric therapies. Observing these competitive tactics reveals critical lessons on how to sustain momentum in an increasingly crowded and innovative market.
This comprehensive research report delivers an in-depth overview of the principal market players in the Bioelectric Medicine market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Aleva Neurotherapeutics SA
- BioMed Central Ltd.
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- CEFALY Technology sprl
- ElectroCore, Inc.
- General Electric Company
- LivaNova PLC
- MED-EL Elektromedizinische Geräte Gesellschaft m.b.H.
- Medtronic PLC
- NeuroSigma, Inc.
- Nevro Corp.
- Oticon Medical A/S by Cochlear Ltd
- Renishaw PLC
- ReShape Lifesciences, Inc.
- Siemens Healthineers AG
- Sonova Holding AG
- Vomaris Innovations, Inc.
Equipping Industry Leaders with Actionable Roadmaps to Accelerate Bioelectric Medicine Adoption, Elevate Operational Excellence, and Simplify Regulatory Pathways
Industry leaders must adopt a proactive, multi-faceted approach to capitalize on the accelerating momentum in bioelectric medicine. First and foremost, establishing robust R&D partnerships with academic institutions and specialty clinics will unlock cutting-edge scientific insights while sharing development risks. In parallel, investing in modular manufacturing platforms and advanced automation technologies can streamline production workflows, mitigate cost volatility associated with tariff impacts, and accelerate scale-up processes.
Stakeholder engagement strategies should prioritize transparent collaboration with regulatory authorities, leveraging early-stage feedback mechanisms to align on clinical endpoints and post-market surveillance requirements. Equally important is the cultivation of real-world evidence initiatives-such as patient registries and digital monitoring programs-that generate longitudinal outcome data and reinforce value propositions in reimbursement discussions. Simultaneously, organizations should refine their commercialization playbooks by integrating value-based pricing frameworks and targeting both traditional hospital settings and emerging homecare channels.
By executing these actionable roadmaps-spanning R&D co-innovation, operational excellence, regulatory alignment, and evidence-driven commercialization-industry players can accelerate adoption, reinforce stakeholder confidence, and secure sustainable growth in this transformative therapeutic frontier.
Detailing Research Methodology Employing Multi-Source Literature Review, Expert Interviews with Quantitative Analysis Delivering Bioelectric Medicine Insights
This analysis is underpinned by a rigorous research methodology that synthesizes insights from multiple data streams and expert perspectives. Initially, an exhaustive literature review encompassing peer-reviewed journals, conference proceedings, and regulatory filings established a foundational understanding of technology trends, clinical endpoints, and regulatory trajectories. Building on this, a series of structured interviews with key opinion leaders-comprising neurologists, cardiovascular specialists, biomedical engineers, and payers-provided nuanced viewpoints on clinical adoption challenges and emerging value drivers.
To complement qualitative inputs, quantitative analysis of device approval timelines, patent portfolios, and public-company financial disclosures was conducted to validate market dynamics and competitive positioning. Data triangulation techniques ensured that findings were cross-verified, minimizing bias and reinforcing the robustness of strategic recommendations. This multi-source approach guarantees that stakeholders receive comprehensive, evidence-based insights into the evolving bioelectric medicine landscape.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Bioelectric Medicine market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Bioelectric Medicine Market, by Technology
- Bioelectric Medicine Market, by Indication
- Bioelectric Medicine Market, by Application
- Bioelectric Medicine Market, by End User
- Bioelectric Medicine Market, by Region
- Bioelectric Medicine Market, by Group
- Bioelectric Medicine Market, by Country
- United States Bioelectric Medicine Market
- China Bioelectric Medicine Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1113 ]
Distilling Critical Findings in Bioelectric Medicine to Illuminate Future Directions, Pathways, and the Promise of Next-Generation Neuromodulation Therapies
In distilling the critical findings of this executive summary, several overarching themes emerge. The convergence of technological innovation, evolving reimbursement frameworks, and heightened patient engagement has propelled bioelectric medicine into a central role within modern therapeutic arsenals. Concurrent regulatory shifts and tariff-driven supply chain dynamics underscore the importance of strategic resilience and adaptive sourcing strategies. Moreover, segmentation analytics reveal distinct opportunities for product differentiation across technology modalities, clinical applications, care settings, and patient indications.
Regionally, the Americas’ established R&D ecosystems, EMEA’s diverse market access pathways, and Asia-Pacific’s manufacturing capabilities collectively shape a complex yet opportunity-rich global landscape. Competitive intelligence on leading device makers illustrates strategic imperatives in portfolio diversification, partnerships, and real-world evidence generation. Finally, the actionable recommendations provide a clear blueprint for industry leaders to accelerate adoption, optimize operational frameworks, and navigate regulatory complexities. Collectively, these insights chart a course for stakeholders to harness the full potential of bioelectric medicine and drive sustained, patient-centered innovation.
Engage with Associate Director Sales & Marketing Ketan Rohom to Unlock Comprehensive Bioelectric Medicine Intelligence and Secure Your Market Research Report
We invite you to engage directly with Associate Director Sales & Marketing Ketan Rohom to discover how this comprehensive bioelectric medicine intelligence can empower your strategic decisions. By establishing a conversation with Ketan, you’ll gain personalized insights into technology adoption trends, regulatory dynamics, and segmentation opportunities tailored to your organization’s objectives. Leveraging his deep domain expertise, you can explore customized advisory services that align with your investment horizons and operational pivots. Moreover, a dialogue with Ketan unlocks access to detailed data supplements, exclusive executive interviews, and scenario planning tools designed to accelerate your go-to-market strategies.
Take the next step toward securing a competitive edge: partner with Ketan Rohom to purchase the full market research report and access an integrated suite of decision-support resources. Empower your team with actionable recommendations, regional analyses, and company profiles that translate complex bioelectric medicine trends into clear, implementable strategies. Reach out today to transform insights into impact and ensure your organization thrives in this rapidly evolving therapeutic frontier.

- How big is the Bioelectric Medicine Market?
- What is the Bioelectric Medicine Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




