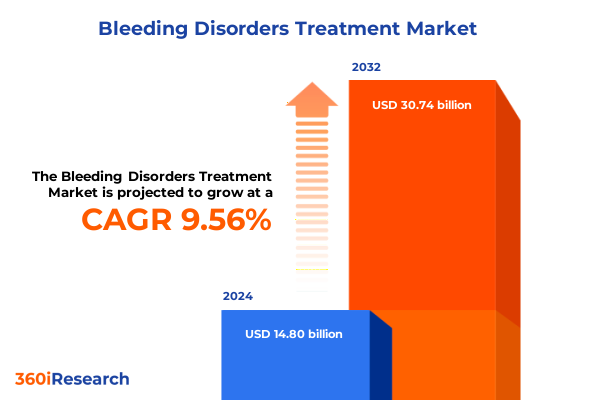
The Bleeding Disorders Treatment Market size was estimated at USD 16.04 billion in 2025 and expected to reach USD 17.38 billion in 2026, at a CAGR of 9.73% to reach USD 30.74 billion by 2032.
Exploring the Dynamics of Bleeding Disorders Treatment Markets and the Critical Drivers Shaping Therapeutic Innovations and Stakeholder Engagement
Bleeding disorders represent a critical area of unmet need within global healthcare, characterized by chronic conditions such as hemophilia A, hemophilia B, and Von Willebrand disease that demand precise and sustained therapeutic intervention. With increasing recognition of these diseases and expanding diagnostic capabilities, stakeholders across pharmaceutical, biotech, and healthcare delivery sectors are intensifying efforts to innovate and improve patient outcomes. Clinicians, payers, and patient advocacy groups collectively emphasize the necessity for safe, effective, and accessible hemostatic therapies that can adapt to the nuanced clinical demands of both acute bleeding episodes and long-term prophylactic regimens.
This executive summary distills a detailed exploration into bleeding disorders treatment, highlighting the intricate interplay between regulatory frameworks, technological advancements, and evolving patient and provider expectations. It lays the groundwork for decision-makers seeking a rigorous understanding of market dynamics, therapeutic breakthroughs, and systemic challenges that shape investment and development priorities. By elucidating critical drivers and identifying key inflection points, this analysis sets the stage for targeted strategic initiatives designed to accelerate innovation, optimize care pathways, and enhance value creation across the bleeding disorders ecosystem.
Identifying Paradigm-Shifting Developments in Research, Policy, and Technology That Are Redefining the Bleeding Disorders Treatment Landscape Globally
In recent years, the bleeding disorders treatment landscape has undergone transformative shifts driven by scientific breakthroughs, policy evolution, and the digitization of healthcare. Gene therapies, which aim to address underlying genetic defects through single-administration interventions, have moved from preclinical promise to regulatory review, prompting discussions on long-term efficacy, safety monitoring, and sustainable pricing models. Concurrently, advances in recombinant technology and plasma-derived manufacturing processes have improved the safety profile and supply stability of clotting factors, enabling more effective prophylactic regimens and reducing the burden of repeated infusions.
Beyond biologics, emerging classes of anti-fibrinolytics and novel desmopressin formulations are reshaping standard of care by offering alternative mechanisms to enhance hemostasis. These innovations have been complemented by digital health platforms that facilitate remote monitoring of bleeding episodes, dosage adherence, and patient-reported outcomes. Telemedicine initiatives, accelerated by the COVID-19 pandemic’s telehealth adoption curve, now play a vital role in chronic disease management, extending specialist expertise to home care settings and specialty clinics alike.
On the policy front, regulatory agencies in the United States and Europe are adapting frameworks to accommodate accelerated approvals and real-world evidence studies. Multi-stakeholder collaborations, including patient advocacy organizations and public payers, are shaping value-based payment models that link reimbursement to long-term clinical benefits. Collectively, these shifts underscore a transition from reactive acute care to proactive, patient-centric treatment paradigms, propelling the bleeding disorders sector toward greater personalization and integrated care delivery.
Unraveling the Comprehensive Effects of United States Tariff Policies Implemented in 2025 on the Supply Chain, Pricing, and Accessibility of Hemostatic Therapies
The imposition of new tariff measures by the United States in 2025 has introduced a complex layer of cost and supply chain considerations for manufacturers and distributors of hemostatic therapies. As many therapeutic proteins and recombinant products rely on cross-border manufacturing and specialized raw material imports, even modest tariff adjustments can reverberate through pricing structures, inventory planning, and contract negotiations with hospitals and retail pharmacies. For manufacturers, these duties have necessitated strategic recalibrations, including reshoring certain production processes, securing alternative sourcing agreements, and exploring tariff mitigation through bonded warehouse operations.
Healthcare providers and payers are also navigating the downstream effects, as increased import costs can translate into elevated reimbursement claims and potential co-payment adjustments for patients. Specialty clinics, which often deliver high-cost biologics under tightly managed care protocols, have become focal points for cost-containment strategies. Distribution partners have responded by optimizing logistics networks, consolidating shipment volumes to qualify for reduced duty thresholds, and leveraging digital freight management tools to maintain service levels while controlling overheads.
Despite these challenges, the cumulative impact of tariffs has also stimulated investments in domestic capacity expansion and public-private partnerships aimed at strengthening supply resilience. By diversifying geographic manufacturing footprints and accelerating technology transfers, industry leaders are positioning themselves to buffer future trade policy shifts. Ultimately, the 2025 tariff environment has galvanized a more agile and vertically integrated bleeding disorders ecosystem-one that balances regulatory compliance with cost-effective delivery of essential therapies.
Gleaning Insights through Segmentation Spanning Product Category, Treatment Modality, Administration Route, User Profile and Disease Indication
A nuanced understanding of market segmentation reveals how therapeutic strategies align with patient needs, provider capabilities, and distribution efficiencies. Within the product type division, anti-fibrinolytics address acute bleeding control while clotting factors-segmented into plasma-derived and recombinant variants-serve both immediate and prophylactic purposes, and desmopressin offers a synthetic alternative for specific indications. Treatment modalities further distinguish on-demand regimens, targeting episodic hemorrhages, from prophylactic approaches designed to preempt bleeding and preserve musculoskeletal health.
Route of administration insights underscore the growing preference for subcutaneous formulations that facilitate at-home self-administration, compared with traditional intravenous infusions conducted in hospital or clinic settings. Oral therapies, while limited to certain drug classes, are gaining traction due to ease of dosing and improved patient adherence. End-user analysis highlights the diverse care environments encompassing home care settings, large hospital systems, and specialty clinics, each with unique logistical requirements, regulatory oversight, and reimbursement pathways. Distribution channel segmentation sees the emergence of e-commerce platforms as a complement to established hospital and retail pharmacy networks, particularly for chronic, self-administered therapies.
Disease indication segmentation underscores the market’s focus on hemophilia A, hemophilia B, and Von Willebrand disease, each presenting distinct clinical profiles and therapeutic challenges. Together, these multifaceted segmentation lenses provide clarity on where investment, innovation, and commercialization efforts should be prioritized to maximize patient benefit and commercial returns.
This comprehensive research report categorizes the Bleeding Disorders Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Treatment Type
- Route Of Administration
- Disease Indication
- Distribution Channel
- End Users
Unveiling Regional Nuances in Hemostatic Therapy across Americas, Europe Middle East & Africa and Asia-Pacific to Highlight Strategic Prospects and Hurdles
Regional dynamics in the bleeding disorders market are shaped by divergent regulatory landscapes, healthcare infrastructure maturity, and reimbursement frameworks. In the Americas, the United States leads with robust R&D investment, a highly concentrated specialty clinic network, and evolving payer models that increasingly favor value-based contracts. Canada’s public healthcare system, while supportive of novel therapies, imposes stringent health technology assessments that affect market entry timing and pricing negotiations.
Europe, the Middle East & Africa region exhibits a complex mosaic of national regulatory authorities and funding mechanisms. Western European countries often serve as launch hubs for innovative biologics, supported by coordinated transnational reimbursement dialogues. Emerging markets in the Middle East and select African nations are expanding screening and treatment capacity, though access remains uneven due to budgetary constraints and limited specialist availability.
In Asia-Pacific, rapid economic growth and increasing healthcare spend are catalyzing market expansion in countries like Japan, China, and Australia. National insurance schemes and domestic manufacturing partnerships are accelerating access to both established clotting factors and cutting-edge gene therapies. However, disparities persist in rural and under-resourced areas, prompting stakeholders to invest in telehealth and community care initiatives. Cross-regional collaborations and technology transfers are further bridging gaps, enabling a more cohesive global approach to bleeding disorders management.
This comprehensive research report examines key regions that drive the evolution of the Bleeding Disorders Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Examining Innovative Pharmaceutical and Biotech Companies Driving Collaborations, Breakthrough Products and Competitive Strategies in Bleeding Disorders Therapies
Leading pharmaceutical and biotechnology companies are driving market transformation through targeted R&D investments, strategic collaborations, and portfolio diversification. Established players leverage decades of expertise in plasma-derived products and recombinant technologies to optimize manufacturing yields and safety profiles. Partnerships with academic institutions and specialty research centers are advancing novel formulations and next-generation gene therapies.
Simultaneously, emerging biotech firms are introducing disruptive platforms such as RNA-based approaches and novel anti-fibrinolytic compounds, catalyzing competition and accelerating innovation cycles. These smaller entities often engage in licensing agreements or co-development partnerships with larger firms to navigate regulatory pathways and scale commercial operations. Additionally, cross-sector alliances with digital health companies are integrating remote monitoring and data analytics into comprehensive care models, enhancing adherence and enabling real-world evidence generation.
Competitive differentiation is increasingly defined by a company’s ability to integrate supply chain resilience, patient support programs, and value-based contracting mechanisms. Strategic acquisitions and geographic expansions are further diversifying pipelines and broadening market access. As a result, the bleeding disorders treatment ecosystem is witnessing a dynamic interplay between legacy expertise and entrepreneurial agility, positioning patients at the center of an increasingly innovative therapeutic landscape.
This comprehensive research report delivers an in-depth overview of the principal market players in the Bleeding Disorders Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Alnylam Pharmaceuticals
- Bayer AG
- Biogen Inc.
- BioMarin Pharmaceutical Inc.
- Bristol-Myers Squibb
- CSL Limited
- F. Hoffmann-La Roche Ltd
- Ferring Pharmaceuticals
- Grifols, S.A.
- Janssen Pharmaceuticals
- Novo Nordisk A/S
- Octapharma AG
- Pfizer Inc.
- Sanofi S.A.
- Sun Pharmaceuticals Pvt. Ltd.
- Swedish Orphan Biovitrum AB
- Takeda Pharmaceutical Company Limited
- Zydus Lifesciences
Actionable Recommendations for Industry Leaders to Improve Patient Outcomes, Streamline Value Chains and Navigate Regulatory and Market Complexities in Bleeding Disorders
Industry leaders should prioritize a multipronged strategy that aligns clinical innovation with commercial execution and policy engagement. First, investing in patient support and education programs can drive adherence, optimize treatment outcomes, and generate robust real-world evidence to inform payer negotiations. Secondly, integrating risk-sharing agreements and outcome-based contracts with distributors and payers will align incentives around long-term therapeutic benefits, mitigating pricing pressures associated with acute cost-containment measures.
Supply chain diversification, including dual sourcing of critical biologic components and expansion of regional manufacturing hubs, will enhance resilience against trade policy fluctuations. Concurrently, adoption of digital platforms for remote monitoring and telehealth consultations can extend specialist reach into home care and rural settings, improving access and reducing overall healthcare costs. Engaging proactively with regulatory authorities to support accelerated approval pathways, coupled with robust post-market safety surveillance, will expedite patient access to breakthrough therapies.
Finally, forging public-private partnerships with government bodies and advocacy organizations can streamline newborn screening programs and educational initiatives, ensuring early diagnosis and treatment initiation. By implementing these targeted actions, industry stakeholders can achieve sustainable growth, elevate patient care standards, and navigate the evolving market landscape with confidence.
Detailing a Robust Mixed-Methods Research Approach Incorporating Expert Interviews, Comprehensive Secondary Data Review and Multi-Stage Validation
The research underpinning this analysis adopted a mixed-methods framework, beginning with in-depth interviews conducted with clinical experts, patient advocacy leaders, supply chain executives, and reimbursement specialists. These primary consultations provided qualitative insights into evolving treatment paradigms, operational challenges, and emerging stakeholder priorities. Secondary data sources, including peer-reviewed journals, regulatory filings, and patent databases, were systematically reviewed to validate market trends and technology developments.
Subsequently, a multi-stage validation process was employed, wherein preliminary findings were presented to an advisory panel comprising hematologists, health economists, and commercial strategists. Feedback loops ensured methodological rigor and practical relevance, refining segmentation schemes and highlighting regional nuances. Statistical triangulation techniques were applied to reconcile quantitative data points with qualitative narratives, enhancing the credibility of insights derived.
Quality assurance protocols, including data integrity checks and peer review processes, reinforced the reliability of conclusions. The combination of expert elicitation, comprehensive data review, and structured validation delivers a robust intelligence platform tailored to inform high-stakes strategic decision-making in the bleeding disorders treatment domain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Bleeding Disorders Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Bleeding Disorders Treatment Market, by Product Type
- Bleeding Disorders Treatment Market, by Treatment Type
- Bleeding Disorders Treatment Market, by Route Of Administration
- Bleeding Disorders Treatment Market, by Disease Indication
- Bleeding Disorders Treatment Market, by Distribution Channel
- Bleeding Disorders Treatment Market, by End Users
- Bleeding Disorders Treatment Market, by Region
- Bleeding Disorders Treatment Market, by Group
- Bleeding Disorders Treatment Market, by Country
- United States Bleeding Disorders Treatment Market
- China Bleeding Disorders Treatment Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1272 ]
Synthesizing Key Insights and Reflecting on Strategic Pathways to Advance Patient Care, Foster Sustainable Growth and Drive Innovation in Bleeding Disorders
In synthesizing the foregoing analysis, it is clear that the bleeding disorders treatment sector stands at a pivotal juncture. Advancements in gene therapy, recombinant technologies, and digital health are converging to redefine care pathways and patient experiences. At the same time, trade policy fluctuations and diverse regional healthcare systems impose operational complexities that demand agile, data-driven strategies.
Strategic focus on segmentation insights-spanning product type, treatment modality, administration route, user environment, and disease indication-will guide resource allocation toward the most impactful therapeutic opportunities. Regional differentiation underscores the importance of tailored market entry and expansion plans, while competitive benchmarking highlights collaboration as a catalyst for innovation.
Ultimately, stakeholders who integrate these insights into cohesive action plans-balancing clinical efficacy, economic value, and regulatory foresight-will be best positioned to deliver transformative patient outcomes and sustained organizational growth in the evolving bleeding disorders treatment landscape.
Empower Your Strategic Decisions in Bleeding Disorders Treatment with Tailored Market Intelligence Services from Our Associate Director of Sales & Marketing
Unlock unparalleled clarity and direction in your strategic initiatives by engaging directly with Ketan Rohom, Associate Director, Sales & Marketing. Through a personalized consultation, you will gain precise guidance on applying the report’s in-depth analysis to your organizational priorities. Discussion topics may include identifying high-potential therapeutic segments, anticipating policy impacts, and optimizing distribution strategies to ensure robust competitive advantage. Reach out today to secure your copy of the comprehensive market research report and transform insights into actionable growth pathways.

- How big is the Bleeding Disorders Treatment Market?
- What is the Bleeding Disorders Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




