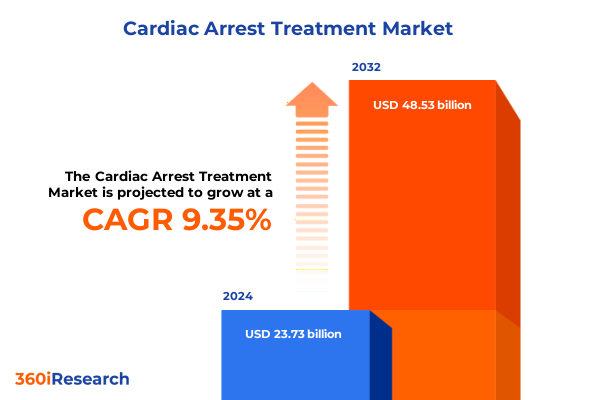
The Cardiac Arrest Treatment Market size was estimated at USD 25.63 billion in 2025 and expected to reach USD 27.70 billion in 2026, at a CAGR of 9.54% to reach USD 48.53 billion by 2032.
Exploring the Critical Foundations and Emergent Dynamics Shaping Cardiac Arrest Treatment in Today’s Rapidly Evolving Healthcare Environment
Introduction
The landscape of cardiac arrest treatment has evolved profoundly, driven by breakthroughs in device technology, pharmacological advances, and a critical need for faster, more effective intervention protocols. As incidences of out-of-hospital cardiac arrest continue to challenge emergency response systems, industry stakeholders are compelled to innovate across clinical, logistical, and regulatory dimensions. The imperative to improve survival rates and neurological outcomes is matched only by the pressing demand to streamline supply chains and adapt to shifting healthcare reimbursement structures. In this context, a holistic examination of prevailing market forces and transformative breakthroughs is essential to inform strategic planning and investment.
This executive summary distills the most consequential developments shaping cardiac arrest intervention strategies, from cutting-edge external circulatory support devices to novel drug therapies that stabilize patients during resuscitation. By synthesizing insights across treatment modalities, end-user environments, distribution pathways, and patient demographics, this overview presents an integrated perspective tailored for senior decision-makers. Moreover, it highlights the cumulative impact of U.S. trade policies and tariff adjustments enacted in 2025, offering a nuanced lens on procurement, cost management, and regional competitiveness. Ultimately, this executive summary aims to equip executives with a clear, forward-looking framework for capitalizing on market opportunities while mitigating emerging risks.
How Technological Innovations and Clinical Protocol Advancements Are Driving Fundamental Transformations in Cardiac Arrest Management Worldwide
Innovations in cardiac arrest management are not merely iterative; they represent a fundamental shift in how clinicians and first responders conceptualize resuscitation efficacy. Technological advances in automated compression systems have improved hemodynamic stability during cardiopulmonary resuscitation, while next-generation defibrillator devices integrate artificial intelligence-enhanced algorithms to optimize shock delivery timing and reduce bystander hesitation. Concurrently, developments in pharmacological protocols-such as refined dosing regimens for amiodarone, epinephrine, and vasopressin-are enhancing survivability and neurological recovery when administered in concert with mechanical support.
Beyond devices and drugs, the standard of care is being redefined through data‐driven performance metrics. Real-time feedback using capnography systems and advanced ECG monitoring enables clinicians to make immediate adjustments, ensuring that chest compressions and ventilations align with best-practice guidelines. Surface cooling and endovascular cooling systems for targeted temperature management are further elevating post-resuscitation care by mitigating ischemia-reperfusion injury. Meanwhile, improvements in wearable cardioverter defibrillators and remote monitoring platforms are shifting early detection and intervention outside traditional hospital settings. These transformative shifts collectively underscore a more integrated, patient-centric approach, where technology and therapy converge to maximize outcomes in time-critical scenarios.
Evaluating the Consequential Effects of United States 2025 Trade Tariffs on Cardiac Arrest Treatment Supply Chains and Device Accessibility Nationwide
Since early 2025, the United States has implemented a series of tariffs impacting the importation of medical devices and essential resuscitation components, with targeted levies on metal composites, circuit boards, and calibration modules commonly used in CPR devices and defibrillators. These trade measures have triggered a ripple effect-manufacturers face increased production costs, distributors must reconfigure procurement strategies, and healthcare providers contend with potential price escalations. Although some cost pressures are being absorbed through margin adjustments, many stakeholders are actively exploring supplier diversification and nearshoring alternatives to maintain competitive pricing and ensure uninterrupted supply.
Moreover, the tariffs have accelerated conversations around domestic manufacturing investments. Enterprises are evaluating greenfield expansion and contract manufacturing partnerships across U.S. states offering incentives for advanced medical equipment production. However, supply chain realignment poses challenges: securing skilled labor, ensuring regulatory compliance, and validating quality control for complex devices like implantable cardioverter defibrillators and endovascular cooling systems. Parallel to these efforts, logistical stakeholders are leveraging digital platforms for improved demand forecasting and inventory optimization, softening the impact of tariff-driven volatility. Consequently, while short-term disruptions persist, the cumulative effect of the 2025 U.S. tariffs may ultimately foster a more resilient, innovation-oriented domestic ecosystem for life-saving cardiac arrest treatments.
Unveiling Insightful Patterns Across Treatment Modalities, End Users, Distribution Channels, and Age Cohorts in Cardiac Arrest Intervention Markets
Insight into market segmentation reveals distinct demand patterns when treatment type, end setting, distribution approach, and patient age are considered in unison. Within external circulatory support offerings, mechanical compression solutions like load-distributing band devices and piston-driven devices are increasingly favored in high-volume hospital environments, whereas pneumatic vest devices demonstrate traction in emergency medical services due to their portability and ease of use. Defibrillator solutions further stratify as automated external defibrillators gain acceptance in ambulatory care centers, implantable cardioverter defibrillators continue to be integral in hospital cardiac care units, and wearable cardioverter defibrillators serve as transitional protection for patients discharged from acute settings.
The pharmacotherapy arena shows amiodarone remaining the cornerstone for refractory arrhythmias, with epinephrine protocols optimized for rapid intravenous administration during resuscitation, and vasopressin emerging as an adjunct option in specialized clinical scenarios. Hypothermia management bifurcates between endovascular cooling systems employed in critical care units and surface cooling approaches used in post-resuscitation wards. Monitoring versatility is evident in capnography systems delivering compliance feedback during pre-hospital CPR while ECG monitors provide continuous cardiac rhythm assessment in hospitals. When distribution channels are examined, offline networks dominate high-urgency acquisitions, whereas online procurement platforms are gaining momentum among home care providers seeking convenience. Age-specific dynamics reveal adult cohorts driving baseline volume, geriatric segments reflecting increased utilization of comprehensive defibrillator and monitoring suites, and pediatric populations prioritizing non-invasive hypothermia and specialized drug dosing considerations. Integrating these segmentation layers elucidates granular insights for tailored product positioning and service deployment.
This comprehensive research report categorizes the Cardiac Arrest Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Age Group
- Distribution Channel
- End User
Assessing Regional Dynamics and Strategic Opportunities Across the Americas, EMEA, and Asia-Pacific in the Cardiac Arrest Treatment Landscape
Regional dynamics in cardiac arrest treatment are shaped by divergent healthcare infrastructures, regulatory pathways, and reimbursement models across the Americas, Europe Middle East & Africa, and Asia-Pacific. In the Americas, robust emergency medical services frameworks and clear reimbursement strategies support rapid adoption of advanced defibrillator devices and mechanical CPR solutions, while public-private collaborations are accelerating post-arrest rehabilitation programs. Conversely, in Europe, Middle East & Africa, regulatory harmonization under initiatives like the European Medical Device Regulation has heightened compliance demands but opened access to larger markets, driving investments in endovascular cooling systems and capnography technologies.
Across Asia-Pacific, a heterogeneous landscape emerges: leading economies are expanding hospital networks and upgrading emergency response capabilities, fostering early adoption of integrated monitoring systems, whereas emerging markets are focused on cost-effective drug therapy protocols and portable defibrillators suited for remote settings. In response, manufacturers are customizing product portfolios and distribution strategies to align with local preferences and purchasing power. Furthermore, cross-regional partnerships and licensing agreements are enabling technology transfers that bolster domestic production capabilities. The nuanced interplay of these factors underscores the importance of region-specific go-to-market strategies, emphasizing regulatory foresight, localized manufacturing considerations, and targeted education campaigns to optimize clinical uptake.
This comprehensive research report examines key regions that drive the evolution of the Cardiac Arrest Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Strategic Collaborators Driving Competitive Differentiation in Cardiac Arrest Treatment Technologies and Services
Leading entities in cardiac arrest treatment are harnessing a blend of organic R&D and strategic alliances to consolidate their market positioning. Several device manufacturers have expanded their portfolios through selective acquisitions of start-ups specializing in AI-driven rhythm recognition and remote patient monitoring, thereby augmenting their software-enabled services. Pharmaceutical developers are forging partnerships with infrastructure providers to co-develop combination therapy kits that integrate drug delivery with mechanical support protocols, enhancing procedural efficiency and clinician confidence.
Collaborative initiatives extend to clinical trial networks and academic consortia aimed at validating next-generation therapies such as novel vasopressor combinations and biocompatible cooling catheters. Meanwhile, distributors are leveraging omnichannel strategies to merge offline sales with e-commerce platforms, offering bundled solutions and subscription-based maintenance contracts for critical care devices. Independent service organizations provide aftermarket support, preventive maintenance, and training modules to ensure optimal device performance and regulatory compliance. Collectively, these efforts reflect a competitive ecosystem characterized by continuous innovation, value-added offerings, and an emphasis on end-to-end solutions that address the evolving demands of cardiac arrest intervention across diverse settings.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cardiac Arrest Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Edwards Lifesciences Corporation
- GE HealthCare Technologies Inc.
- Johnson & Johnson
- Koninklijke Philips N.V.
- LivaNova PLC
- Medtronic, Inc.
- Nihon Kohden Corporation
- Siemens Healthineers AG
- Stryker Corporation
- Terumo Corporation
- ZOLL Medical Corporation
Strategic Imperatives and Practical Pathways for Industry Leaders to Enhance Growth, Resilience, and Patient Outcomes in Cardiac Arrest Response
To capitalize on emergent opportunities, industry leaders should prioritize investments in integrated platform development, combining mechanical resuscitation devices with AI-enabled monitoring and drug delivery modules. By fostering cross-functional teams that span engineering, clinical affairs, and regulatory expertise, organizations can accelerate time-to-market for solution bundles optimized for distinct care settings. Additionally, localizing manufacturing and assembly operations within key regions will mitigate tariff impacts and strengthen supply chain resilience, ensuring that critical devices remain accessible during geopolitical disruptions.
Collaboration with emergency medical services and hospital systems is essential for co-creating training programs and real-world evidence studies that validate product performance under actual use conditions. Executives should explore subscription-based models for equipment maintenance and software updates, generating recurring revenue while enhancing customer satisfaction. In parallel, targeted outreach to pediatric and geriatric specialist networks will uncover niche clinical needs and facilitate product adaptations that improve patient-centric outcomes. Finally, leveraging digital marketing and telehealth channels to engage end users directly can drive adoption, gather actionable feedback, and position organizations as thought leaders in cardiac arrest treatment innovation.
Detailing the Rigorous Research Framework, Data Collection Techniques, and Analytical Processes Underpinning the Cardiac Arrest Treatment Market Study
The research underpinning this executive summary combines qualitative insights from in-depth interviews with leading clinicians, industry executives, and regulatory authorities alongside quantitative data aggregated from proprietary databases and public sources. Primary research included structured discussions with emergency department heads, EMS coordinators, and biomedical engineers to capture firsthand perspectives on adoption challenges, performance benchmarks, and clinical workflow integration.
Secondary research leveraged peer-reviewed journals, regulatory filings, and trade publications to validate market trends and technological advancement timelines. Data triangulation was employed to reconcile inconsistencies between sources, ensuring robust conclusions. Analytical processes comprised cross-segmentation analysis to identify correlations between treatment types, settings, and demographic factors, supplemented by scenario planning to assess the impact of U.S. tariff adjustments on supply chain configurations. Quality assurance measures included peer reviews by external subject-matter experts and iterative validation cycles, ensuring that findings are both accurate and actionable for strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cardiac Arrest Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cardiac Arrest Treatment Market, by Treatment Type
- Cardiac Arrest Treatment Market, by Age Group
- Cardiac Arrest Treatment Market, by Distribution Channel
- Cardiac Arrest Treatment Market, by End User
- Cardiac Arrest Treatment Market, by Region
- Cardiac Arrest Treatment Market, by Group
- Cardiac Arrest Treatment Market, by Country
- United States Cardiac Arrest Treatment Market
- China Cardiac Arrest Treatment Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1272 ]
Summarizing Critical Takeaways and Future Outlook for Stakeholders Navigating the Evolving Landscape of Cardiac Arrest Treatment Solutions
In synthesizing the critical findings, several themes emerge as central to the future of cardiac arrest treatment: the convergence of mechanical and pharmacological interventions into coherent, data-driven protocols; the imperative for agile supply chain architectures in response to policy shifts; and the strategic value of regionally tailored go-to-market approaches. Leaders who embrace integrated solutions and foster collaboration across clinical, manufacturing, and distribution domains will be best positioned to drive growth and deliver superior patient outcomes.
Looking ahead, continued innovation in AI-driven monitoring, targeted temperature management, and patient engagement platforms will redefine standards of care. Stakeholders must remain vigilant to evolving reimbursement landscapes and regulatory developments while leveraging real-world evidence to substantiate clinical and economic value. By aligning research and product development investments with the nuanced segmentation insights and regional dynamics outlined here, organizations can sustainably navigate complexity and capitalize on expanding opportunities in the dynamic cardiac arrest treatment ecosystem.
Engage with Associate Director Ketan Rohom to Unlock Comprehensive Insights and Strategic Advantage in the Cardiac Arrest Treatment Market Study
If you are seeking to gain an in-depth, data-driven understanding of the cardiac arrest treatment market-and transform strategic decision-making into measurable growth-connect directly with Associate Director, Sales & Marketing Ketan Rohom. With his deep industry expertise and proven track record in delivering actionable insights, Ketan can guide you through the nuances of market dynamics, competitive positioning, and emerging opportunities. By leveraging his bespoke advisory approach, you will receive tailored recommendations and priority access to proprietary intelligence that can accelerate your organizational goals. Reach out today to discuss how a full market research package can empower you to navigate regulatory complexities, optimize product portfolios, and capitalize on high-impact segments with confidence and agility

- How big is the Cardiac Arrest Treatment Market?
- What is the Cardiac Arrest Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




