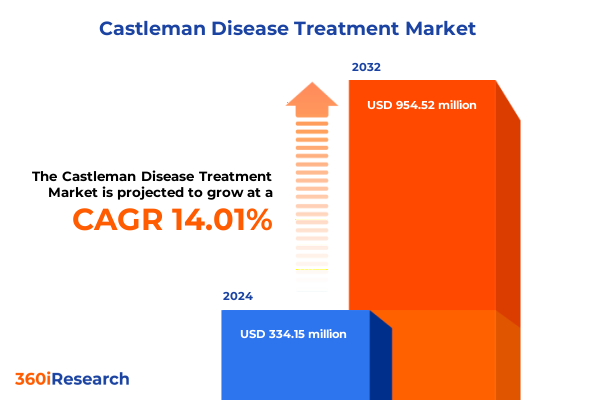
The Castleman Disease Treatment Market size was estimated at USD 380.66 million in 2025 and expected to reach USD 431.11 million in 2026, at a CAGR of 14.03% to reach USD 954.52 million by 2032.
Deep Dive into Castleman Disease: Exploring Its Pathophysiology, Clinical Presentations, and the Unmet Needs Fueling Therapeutic Innovation
Castleman disease represents a spectrum of rare lymphoproliferative disorders distinguished by distinctive histopathological features and variable clinical presentations, necessitating nuanced treatment approaches. In unicentric Castleman disease, a single lymph node region exhibits abnormal proliferation of immune cells and is often amenable to curative surgical excision. In contrast, multicentric Castleman disease involves widespread lymphadenopathy accompanied by systemic inflammation, with idiopathic multicentric Castleman disease (iMCD) further stratified into HHV-8-associated or HHV-8-negative forms based on viral etiology. A newly described oligocentric subtype highlights presentations that fall between unicentric and multicentric patterns, emphasizing the heterogeneity within this rare disease category.
At the core of Castleman disease pathogenesis lies dysregulated interleukin-6 signaling, driving aberrant immune activation and pronounced inflammatory cascades. Elevated inflammatory biomarkers such as C-reactive protein and erythrocyte sedimentation rate often correlate with disease severity. Lymph node histopathology reveals hyaline-vascular, plasmacytic, or mixed subtypes, reflecting underlying immunopathological differences. This molecular understanding has catalyzed the development of targeted immunotherapies that directly neutralize IL-6 or block its receptor, ushering in a new era of precision treatment.
Clinically, patients with iMCD can present with a constellation of constitutional symptoms, including drenching night sweats, unintentional weight loss, fatigue, and fevers. Organ dysfunction may emerge as renal impairment, effusions, or cytopenias, complicating management and often requiring multidisciplinary care. The iMCD-TAFRO subtype, characterized by thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly, exemplifies a severe phenotype warranting aggressive intervention. The rarity of the condition, estimated at 1,000 to 1,500 cases per year in the United States, underscores the limited physician experience and the critical need for consensus clinical guidelines.
Despite the approval of siltuximab as the first FDA-authorized treatment for iMCD and the availability of off-label IL-6 receptor inhibitors, nearly half of patients fail to achieve sustained responses to first-line therapy. Those with refractory disease often require high-dose corticosteroids, chemotherapies, or combination regimens incorporating rituximab, sirolimus, or immunomodulatory agents. The absence of standardized second-line pathways and the disease’s life-threatening potential generate a significant unmet need for innovative therapies and optimized treatment algorithms.
Transformative Momentum in Castleman Disease Therapy: From Cytotoxic Regimens to Precision Immunomodulation and Molecular Diagnostics
The therapeutic paradigm for Castleman disease has undergone a profound transformation, shifting away from broad-spectrum cytotoxic and steroid-based regimens toward highly targeted immunomodulatory strategies. Historically, surgical excision represented the cornerstone for unicentric presentations, while systemic corticosteroids and generalized chemotherapy provided only transient relief for multicentric cases. Advances in molecular pathology have enabled the identification of interleukin-6 as a key driver of disease pathobiology, leading to the advent of monoclonal antibodies that neutralize IL-6 or its receptor with precision previously unattainable by traditional therapies.
Regulatory approvals of siltuximab for iMCD and the integration of tocilizumab in regions where siltuximab is unavailable have established a new standard of care grounded in evidence-based, consensus-driven guidelines. The Castleman Disease Collaborative Network convened international experts to develop formal treatment algorithms that stratify therapy according to disease severity, endorsing anti-IL-6 monoclonal antibodies with or without tailored corticosteroid use for nonsevere disease, and recommending combination chemotherapy for severe nonresponders within days of inadequate response.
Simultaneously, diagnostic criteria have been refined to encompass newly recognized subtypes such as oligocentric Castleman disease and the TAFRO variant of iMCD, which exhibit unique clinical and histologic profiles. Algorithmic approaches integrating imaging modalities, comprehensive laboratory assessments, and strict exclusion criteria for viral, autoimmune, and malignant mimics bolster diagnostic accuracy and timely therapeutic intervention. These advances in molecular diagnostics and classification frameworks are critical to matching patients with the most effective targeted treatments while minimizing exposure to unnecessary systemic toxicity.
Looking forward, the therapeutic landscape continues to evolve with clinical investigation of next-generation immunomodulators and kinase inhibitors, including sirolimus, ruxolitinib, and bortezomib, which offer potential benefits for anti-IL-6 refractory patients. Early-phase studies exploring combination regimens and novel biologics, as well as repurposed agents from related immunological and oncological indications, promise to expand the arsenal of precision therapies. This pipeline reflects a commitment to addressing both the biological complexity of Castleman disease and the pressing unmet needs of patients who do not benefit from existing IL-6 targeted strategies.
Assessing the Ripple Effects of 2025 U.S. Tariff Policies on Castleman Disease Treatment Access, Supply Chains, and Cost Structures
The imposition of broad‐based tariffs on healthcare imports beginning April 5, 2025, has introduced new complexities to the pharmaceutical supply chain and cost structure for Castleman disease therapies. A global 10% tariff on active pharmaceutical ingredients, diagnostic reagents, and medical devices intended to incentivize domestic manufacturing has inadvertently elevated baseline production costs, particularly for rare disease biologics which rely on specialized raw materials.
U.S. tariffs on Chinese and Indian APIs have reached punitive levels of up to 245%, significantly affecting the affordability and availability of generic medications and biologic drug components. Since a substantial portion of critical raw materials for both small‐molecule chemotherapy and monoclonal antibody production is sourced from Asia, manufacturers are facing immediate cost inflations that are only marginally offset by limited supplier concessions. This dynamic exacerbates pricing pressures on therapies such as siltuximab, tocilizumab, and adjunctive corticosteroids, with downstream implications for treatment adherence and patient access.
Biopharmaceutical companies are absorbing a significant share of the tariff burden to forestall an outright pass‐through to patients and preserve stakeholder goodwill. Major players, including AstraZeneca, Pfizer, and Johnson & Johnson, are accelerating domestic manufacturing investments, with AstraZeneca announcing a $50 billion U.S. capital commitment by 2030 to expand biologics and cell therapy capacity. This repositioning aims to mitigate future tariff impacts and reinforce supply chain resilience in the face of ongoing geopolitical volatility.
However, the longer‐term consequences for patient health equity are profound. With generic alternatives-crucial for cost containment in supportive therapies like corticosteroids-facing attrition due to unmanageable input costs, low‐income and underinsured populations bear the greatest risk of treatment discontinuity. Healthcare providers and payers are compelled to reexamine formulary strategies, patient support programs, and inventory management protocols to sustain access amid an evolving tariff landscape.
In response, stakeholders are diversifying raw material sourcing away from high‐tariff jurisdictions, exploring regional suppliers, and implementing advanced procurement models that leverage predictive analytics. While these measures herald a more distributed and agile supply chain, they also extend lead times and require robust quality assurance frameworks to ensure consistent therapeutic integrity for vital treatments in Castleman disease management.
Unraveling Key Segmentation Dynamics: How Treatment Modalities, Therapy Lines, Distribution Channels, and End Users Shape Castleman Disease Management
In the treatment type domain, the Castleman disease landscape encompasses conventional chemotherapeutic approaches alongside targeted monoclonal antibodies and corticosteroids. Chemotherapy regimens serve as monotherapy or combination therapy options for refractory patients, while the anti-interleukin-6 antibodies siltuximab and tocilizumab offer superior tolerability and symptom control. Corticosteroid usage, including standardized dexamethasone and prednisone protocols, continues as an adjunctive measure to rapidly quell inflammatory flares. These layered modalities enable clinicians to tailor interventions based on disease severity and individual patient responsiveness, reflecting the nuanced segmentation of therapeutic mechanisms.
The line of therapy segmentation further distinguishes first-line from second-line and later-line interventions, with initial treatment strategies favoring anti-IL-6 monoclonal antibodies administered as monotherapy or in combination with corticosteroids. Upon inadequate response, second-line protocols introduce rituximab and immunosuppressive or immunomodulatory agents such as sirolimus or thalidomide, chosen to address persistent cytokine dysregulation. Later lines may integrate combination chemotherapy regimens to achieve more aggressive cytoreduction, underscoring the importance of sequential planning to optimize outcomes while managing cumulative toxicity.
Distribution channel segmentation plays a pivotal role in therapy accessibility, with traditional brick-and-mortar specialty pharmacies and hospital infusion centers coexisting alongside burgeoning digital pharmacy platforms. Online specialty pharmacies are increasingly enabling home delivery of monoclonal antibody therapies, supported by refrigerated logistics solutions, while offline channels maintain critical in-person infusion services and real-time clinician engagement. This dual-channel ecosystem enhances patient convenience and continuity of care, particularly valuable for those with mobility or transportation constraints.
End users in Castleman disease treatment encompass home care settings, hospitals, and specialty clinics. Within home care, self-administration of subcutaneous agents and visiting nurse services facilitate outpatient management of stable patients, reducing the need for hospital visits. Hospital settings provide critical infrastructure for initial high-intensity therapies and acute management of severe flares, whereas specialty clinics offer longitudinal care models integrating multidisciplinary support and clinical trial access. Understanding these end-user environments informs targeted deployment of resources and patient support programs aligned with specific care pathways.
This comprehensive research report categorizes the Castleman Disease Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Line Of Therapy
- Distribution Channel
- End User
Deciphering Regional Variations: Market Characteristics and Treatment Adoption Trends Across the Americas, EMEA, and Asia-Pacific for Castleman Disease
The Americas region serves as the largest base for Castleman disease treatment, driven by early regulatory approvals of siltuximab in 2014 and robust reimbursement frameworks in the United States. The presence of established biopharmaceutical manufacturing infrastructure, coupled with patient advocacy networks, supports rapid clinical trial initiation and broad access to advanced therapies. Latin American markets are gradually expanding, though heterogeneity in healthcare funding and diagnostic capacity influences therapy adoption rates and necessitates tailored market entry strategies to address regional reimbursement and regulatory nuances.
In Europe, the Middle East & Africa region, regulatory harmonization under the European Medicines Agency has facilitated cross-border access to tocilizumab and siltuximab, with localized health technology assessments guiding pricing and reimbursement decisions. Markets such as Germany, the United Kingdom, and France exhibit higher per-capita utilization of targeted therapies, while emerging markets in Eastern Europe and the Middle East seek improved diagnostic networks and inclusion of Castleman disease in rare disease policies. Collaboration with regional centers of excellence and alignment with national rare disease plans represent critical paths to optimizing care delivery across this diverse region.
Asia-Pacific is characterized by dynamic growth and regulatory expansion, with Japan endorsing tocilizumab for iMCD since 2013 and China securing NMPA approval for siltuximab in late 2021. These developments underscore the region’s increasingly sophisticated rare disease ecosystem. Investment in local clinical trials and manufacturing partnerships, as demonstrated by collaborations between EUSA Pharma and BeiGene, highlight APAC’s strategic importance. Other regional players, including South Korea, Australia, and India, are progressively integrating advanced immunotherapies into standard treatment algorithms, supported by nascent patient registries and growing clinician expertise.
This comprehensive research report examines key regions that drive the evolution of the Castleman Disease Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Industry Leaders and Innovators: Strategic Initiatives and Pipeline Progress of Major Biopharmaceutical Companies in Castleman Disease Treatment
EUSA Pharma leads in the proprietary commercialization of siltuximab, the only FDA-approved therapy for idiopathic multicentric Castleman disease in the United States and Europe. The company’s strategic acquisition of global rights from Janssen in 2018 and its subsequent partnership with BeiGene for Greater China underscore its commitment to scaling access across critical markets. Ongoing post-approval clinical studies evaluate siltuximab’s broader immunomodulatory potential, reflecting robust lifecycle management and geographic expansion efforts.
Janssen, a subsidiary of Johnson & Johnson, continues to influence the Castleman disease space through its early development of siltuximab and extensive proprietary research on IL-6 pathway biology. As a key innovator in monoclonal antibody therapeutics, Janssen’s ongoing pipeline exploration includes second-generation IL-6 antagonists and novel antibody formats aimed at improving pharmacokinetic profiles and reducing immunogenicity risks.
Roche, via its Actemra (tocilizumab) franchise, holds a leadership position in IL-6 receptor blockade across multiple indications, including Castleman disease in Japan. The company’s expansive global footprint and dual intravenous and subcutaneous formulations offer flexible dosing paradigms. Biosimilar entrants such as Avtozma, approved in early 2025, are poised to introduce competitive pricing dynamics while preserving quality and safety standards.
Emerging players such as Novartis, Takeda, and Incyte are advancing complementary modalities-mTOR inhibition, proteasome blockade, and JAK-STAT pathway targeting respectively-through off-label and investigational applications for refractory cases. These efforts reflect a broader industry realignment, with both large pharmaceutical companies and biotech innovators converging on the Castleman disease indication to address residual unmet clinical needs.
This comprehensive research report delivers an in-depth overview of the principal market players in the Castleman Disease Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Amgen Inc.
- Biogen Inc.
- Bristol-Myers Squibb Company
- Cylene Pharmaceuticals, Inc.
- EUSA Pharma Ltd
- Every Cure Foundation
- F. Hoffmann-La Roche Ltd
- Genentech, Inc.
- Incyte Corporation
- Janssen Sciences Ireland UC
- Novartis AG
- Pfizer Inc.
- Recordati S.p.A
- Regeneron Pharmaceuticals, Inc.
- Sandoz International GmbH
- Senhwa Biosciences, Inc.
- Summit Therapeutics Inc.
- Swedish Orphan Biovitrum AB
- Takeda Pharmaceutical Company Limited
Actionable Strategies for Industry Stakeholders: Enhancing R&D Collaboration, Supply Chain Resilience, and Patient-Centric Programs in Castleman Disease
To address persistent gaps in therapeutic response and patient access, industry leaders should prioritize collaborative research consortia that unite academic centers, patient advocacy groups, and biotech startups. Shared data platforms can accelerate biomarker discovery and streamline trial enrollment, fostering adaptive study designs that reflect real-world heterogeneity and facilitate incremental innovation. Establishing centers of excellence with standardized diagnostic and treatment protocols will enhance clinical outcomes and support evidence generation for novel regimens.
Supply chain resilience must be bolstered through strategic diversification of API sourcing and the development of regional manufacturing hubs. Stakeholders should explore public-private partnerships to underwrite capacity expansions and ensure contingency planning for future tariff shocks. Integrating AI-powered demand forecasting and cold-chain monitoring technologies will optimize inventory management and minimize disruptions for temperature-sensitive biologic therapies.
Enhancing patient-centric programs, including expanded home infusion services and digital adherence monitoring, is essential for improving treatment continuity and quality of life. Industry leaders ought to collaborate with payers to design value-based contracting models that align reimbursement with demonstrated patient outcomes. Additionally, the adoption of telehealth and e-pharmacy channels can extend specialist expertise into underserved areas, mitigating geographical barriers to care.
Advocating for policy reforms that exempt essential biologic therapies from punitive tariffs will alleviate cost pressures and safeguard health equity. Engaging regulatory bodies to streamline orphan drug designation pathways and foster accelerated review for breakthrough therapies will further incentivize investment in rare disease innovation.
Robust Research Methodology Framework: Integrating Primary Clinician Insights, Comprehensive Literature Reviews, and Multisource Data Triangulation
This analysis integrates primary qualitative interviews with hematologists, immunologists, and rare disease program directors across North America, Europe, and Asia-Pacific, conducted through structured discussion guides to capture unmet needs and evolving clinical practices. Secondary research synthesized peer-reviewed literature, regulatory documents, and conference proceedings from authoritative sources such as Blood, NORD, and PMC. All data underwent rigorous cross-validation using triangulation methods to reconcile discrepancies and ensure reliability.
Proprietary databases tracking clinical trial pipelines and patent landscapes were leveraged to identify emerging therapeutic candidates and strategic partnerships. Multi-stakeholder workshops facilitated expert consensus on segmentation frameworks and regional assessments, enabling a nuanced understanding of market dynamics. Financial analyses incorporated publicly disclosed investment commitments, corporate filings, and trade policy impacts to quantify tariff-induced cost variations.
Standardized analytical models were applied to evaluate channel performance, therapy adoption rates, and competitive positioning. Quality control measures, including peer review by subject-matter experts and adherence to established methodological guidelines for rare disease research, underpin the robustness of findings. Ethical compliance was maintained throughout the research process, with informed consent obtained for all primary interviews and adherence to data privacy regulations.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Castleman Disease Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Castleman Disease Treatment Market, by Treatment Type
- Castleman Disease Treatment Market, by Line Of Therapy
- Castleman Disease Treatment Market, by Distribution Channel
- Castleman Disease Treatment Market, by End User
- Castleman Disease Treatment Market, by Region
- Castleman Disease Treatment Market, by Group
- Castleman Disease Treatment Market, by Country
- United States Castleman Disease Treatment Market
- China Castleman Disease Treatment Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1431 ]
Synthesis and Implications: Concluding Insights on Therapeutic Evolution and Strategic Imperatives for the Future of Castleman Disease Management
The Castleman disease treatment landscape stands at a pivotal juncture, characterized by the maturation of IL-6 targeted therapies and the emergence of novel immunomodulatory agents poised to address refractory cases. Evolving clinical guidelines, anchored in consensus frameworks, provide a roadmap for personalized treatment stratification and underscore the importance of early diagnostic precision. While siltuximab and tocilizumab have redefined first-line management, the unmet clinical need among nonresponders continues to drive innovation in areas such as JAK-STAT inhibition, proteasome blockade, and combination regimens.
Geopolitical shifts and U.S. tariff policies have prompted a reevaluation of global supply chains, catalyzing investments in domestic manufacturing and strategic API diversification. These developments are reshaping cost structures and influencing payer negotiations, with long-term implications for patient access and health equity. Concurrently, segmentation insights reveal the critical interplay of treatment types, therapy lines, and distribution channels in tailoring interventions to diverse care settings, from home infusion programs to specialized clinic networks.
Regional trends highlight the Americas as a leader in market adoption, EMEA’s alignment of reimbursement frameworks with rare disease initiatives, and APAC’s rapid regulatory advancements in China and Japan. Industry leaders, including EUSA Pharma, Roche, and Janssen, are leveraging acquisitions and partnerships to expand geographic reach, while emerging biotechs and repurposed immunomodulators promise to fill residual gaps. Collaboration across public, private, and patient organization sectors will be essential to sustain momentum.
Looking ahead, strategic imperatives center on enhancing R&D collaborations, fortifying supply chain resilience, and championing patient-centric care innovations. By aligning policy advocacy with operational excellence and scientific rigor, stakeholders can navigate the complex market landscape and ensure that advances in Castleman disease therapeutics translate into tangible benefits for patients worldwide.
Connect with Associate Director Ketan Rohom to Unlock Comprehensive Castleman Disease Treatment Intelligence and Secure Your Market Research Report Today
Elevate your Castleman disease treatment strategies with a comprehensive market intelligence report tailored to your business objectives by connecting directly with Ketan Rohom, Associate Director of Sales & Marketing. Gain exclusive insights into transformative therapeutic shifts, tariff impacts, segmentation breakdowns, regional dynamics, and company profiles that will inform your next strategic decisions. Reach out today to secure your copy of the in-depth report and equip your organization with the data and analysis needed to outpace competitors and better serve the rare disease community.

- How big is the Castleman Disease Treatment Market?
- What is the Castleman Disease Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




