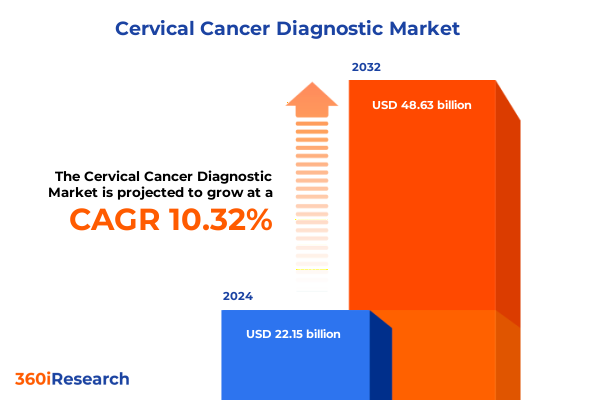
The Cervical Cancer Diagnostic Market size was estimated at USD 24.46 billion in 2025 and expected to reach USD 27.02 billion in 2026, at a CAGR of 10.31% to reach USD 48.63 billion by 2032.
Unveiling the Critical Role of Early Detection and Cutting-Edge Technologies Reshaping the Future of Cervical Cancer Diagnostics Across Healthcare Ecosystems
Cervical cancer remains a significant global health challenge despite advances in preventive measures. In the United States alone, approximately 14,100 women receive a cervical cancer diagnosis each year, emphasizing the persistent need for effective and accessible diagnostic strategies. The World Health Organization set an ambitious goal to eliminate cervical cancer as a public health problem by 2030, underscoring the urgency of expanding screening coverage and early detection efforts.
Against this backdrop, the cervical cancer diagnostics field is experiencing a rapid transformation driven by technological innovation and evolving clinical guidelines. Traditional cytology-based approaches are now complemented by DNA-based HPV assays, digital pathology, and artificial intelligence–enabled colposcopes that enhance sensitivity and streamline workflows. Simultaneously, self-sampling kits and telemedicine platforms are breaking down geographic and cultural barriers to screening, promising greater patient engagement and higher compliance rates.
This executive summary offers an expert overview of the landscape, detailing the transformative shifts powering innovation, the cumulative impact of newly introduced US tariffs, deep segmentation and regional insights, and an analysis of leading companies. It concludes with strategic, actionable recommendations for industry leaders, a concise explanation of the research methodology employed, and a compelling call to action to procure the full report. Readers can leverage these insights to navigate challenges and capitalize on emerging opportunities in cervical cancer diagnostics.
Charting Unprecedented Shifts Driven by AI Integration, Molecular Sequencing Innovations, and Patient-Centric Screening Models in Cervical Cancer Diagnostics
The cervical cancer diagnostics landscape is undergoing unprecedented change as a result of converging technological breakthroughs and patient-centric models. High-resolution digital cytology platforms now harness deep-learning algorithms to identify pre-cancerous lesions with greater precision than ever before. These AI-driven systems, recently cleared by regulatory authorities, reduce manual review times and increase throughput, enabling laboratories to process larger volumes of samples with consistent accuracy.
Meanwhile, molecular innovations such as next-generation sequencing (NGS) are expanding the scope of HPV genotyping, allowing for comprehensive detection of multiple high-risk strains in a single assay. This multiplex capability not only enhances diagnostic sensitivity but also supports personalized risk stratification, guiding clinicians toward optimal follow-up strategies. At the same time, novel CRISPR-based diagnostic prototypes have demonstrated potential for rapid, low-cost HPV detection at the point of care, paving the way for democratized screenings in resource-limited settings.
Concurrently, the rise of self-collection solutions and telemedicine integration is transforming patient engagement. By enabling individuals to provide cervicovaginal samples in the privacy of their homes, these approaches overcome social and logistical barriers to traditional screening. Early pilot programs have shown increased participation rates, particularly among underserved populations, suggesting that patient-centric delivery models will be essential to achieving global elimination targets. As these shifts converge, stakeholders must adapt their strategies to align with the new paradigm of precision, accessibility, and digital connectivity.
Assessing the Cumulative Impact of 2025 US Tariff Measures on Supply Chains, Cost Structures, and Strategic Resilience in Cervical Cancer Diagnostics
In 2025, the introduction of broad-based U.S. import tariffs has created significant headwinds for the cervical cancer diagnostics supply chain. A uniform 10% levy on most imported goods, coupled with higher duties on certain trading partners, threatens to elevate the cost of key diagnostic components and instruments. While some segments, such as domestically manufactured HPV tests, remain largely insulated, many instruments and specialized reagents sourced globally face potential price increases.
Medical device makers anticipate these tariffs could translate into hundreds of millions of dollars in additional costs by year-end. Companies with complex, multinational supply chains have already begun evaluating near-shoring or diversification strategies to mitigate exposure. However, establishing new manufacturing sites or qualifying alternative suppliers requires substantial time and capital, creating interim risks of supply shortages and production delays during the transition period.
Healthcare providers may encounter reduced predictability in procurement budgets, with hospitals and clinics potentially deferring equipment upgrades or scaling back procurement volumes. To safeguard patient access, industry groups and trade associations are advocating for targeted exemptions on critical diagnostic tools. In parallel, diagnostic laboratories are exploring bulk purchasing agreements and extended stockpiling to buffer against cost volatility. Despite immediate challenges, these measures underscore the importance of strategic resilience and proactive supply chain management in maintaining uninterrupted diagnostic services.
Unearthing Key Insights Across Product Types, Test Modalities, Technological Platforms, End Users, Sample Types, Distribution Channels in Cervical Cancer Diagnostics
A nuanced understanding of market segmentation is essential for strategic positioning within the cervical cancer diagnostics arena. Instruments and kits & reagents constitute the foundational product types, with instruments subdivided into colposcopes, microscopes, and PCR instruments that serve core diagnostic functions. Kits & reagents encompass cytology kits, HPV DNA test kits, and VIA kits, each designed to address specific screening modalities with tailored assay chemistries and workflows.
From a test type perspective, the market spans cytology, HPV DNA testing, and visual inspection techniques. Cytology maintains its role as a traditional screening method, while HPV DNA testing increasingly serves as the preferred primary screening tool for women over 30 due to its higher sensitivity. Visual inspection remains prevalent in low-resource settings where immediate, low-cost assessments are vital.
Technological segmentation highlights hybrid capture, next-generation sequencing, and polymerase chain reaction platforms. Hybrid capture remains a workhorse in high-volume laboratories, while NGS and PCR technologies continue to drive precision and multiplexing capabilities. End users range from diagnostic laboratories and hospitals to research institutes and specialty clinics, each with distinct throughput requirements and regulatory considerations. Sample types further delineate the market between conventional smear and liquid-based cytology, with the latter gaining favor for improved cell preservation and integration into molecular workflows. Distribution channels include direct sales, online channels, and third-party distributors, reflecting diverse procurement preferences and geographic reach. This multifaceted segmentation framework enables stakeholders to tailor offerings and prioritize investments according to evolving clinical and operational needs.
This comprehensive research report categorizes the Cervical Cancer Diagnostic market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Test Type
- Technology
- Sample Type
- Distribution Channel
- End User
Revealing Key Regional Insights: Navigating Americas, Europe Middle East & Africa, and Asia Pacific Dynamics in Cervical Cancer Diagnostics Markets
Regional dynamics play a pivotal role in shaping the adoption and development of cervical cancer diagnostics. In the Americas, robust reimbursement pathways, established laboratory infrastructures, and favorable regulatory environments have accelerated the uptake of advanced HPV testing solutions. The US Preventive Services Task Force’s endorsement of DNA-based primary screening has further catalyzed market growth. At the same time, Latin American nations are investing in mobile screening programs and public-private partnerships to expand coverage in underserved communities.
In Europe, Middle East & Africa (EMEA), national screening programs and stringent regulatory frameworks drive a focus on harmonized quality standards. The UK’s IMPACT trial demonstrated the efficacy of home-based self-collection approaches, influencing policy discussions across the European Union. Importantly, emerging markets in Africa are benefitting from WHO-led initiatives that subsidize self-sampling kits and integrated diagnostic platforms to bridge healthcare accessibility gaps.
Asia-Pacific presents a highly heterogeneous landscape, with countries such as India and China experiencing rapid modernization of diagnostic capabilities. In April 2025, India introduced indigenously developed HPV test kits under a government partnership program, marking a significant step toward local manufacturing and cost containment. Meanwhile, Chinese firms are stockpiling materials and exploring domestic testing alternatives to mitigate the impact of broader trade tensions, underscoring the region’s resilience and strategic agility.
This comprehensive research report examines key regions that drive the evolution of the Cervical Cancer Diagnostic market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Unveiling Key Company Insights: Evaluating Strategic Leadership, Innovative Offerings, and Competitive Positioning of Major Players in Cervical Cancer Diagnostics
The competitive landscape in cervical cancer diagnostics is defined by several industry leaders that combine global reach with deep portfolios. Roche continues to command a significant presence through its cobas HPV test and self-collection solutions, leveraging extensive clinical validation and regulatory approvals to maintain market leadership. Hologic’s Aptima system remains a strong contender, particularly in North America, supported by its high-throughput platforms and proprietary assay technologies.
Abbott Laboratories, known for its Alinity m HR HPV Assay, has demonstrated consistent revenue growth by expanding HPV testing into emerging markets. Becton Dickinson and Company has differentiated itself through strategic partnerships and integrated digital pathology offerings that streamline workflow efficiencies. Qiagen’s Digene and Cervista assays maintain relevance through targeted partnerships and continuous assay optimization.
Emerging players such as Mammoth Biosciences are challenging incumbents with CRISPR-based diagnostics that promise rapid turnaround times and point-of-care utility. Meanwhile, diagnostic giants like Quest Diagnostics and Labcorp leverage their extensive laboratory networks and patient-service centers to broaden access to co-testing solutions. This diverse competitive set, ranging from established multinational corporations to agile biotechnology start-ups, fosters an environment of rapid innovation and strategic collaboration.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cervical Cancer Diagnostic market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Advaxis Inc.
- Arbor Vita Corporation
- Beckman Coulter Inc. by Danaher Corporation
- Becton, Dickinson and Company
- Bio Farma
- Bio-Rad Laboratories, Inc.
- Bristol-Myers Squibb Company
- Cardinal Health, Inc.
- CooperSurgical, Inc.
- Dr Lal PathLabs Pvt. Ltd.
- DYSIS Medical Inc.
- F. Hoffmann-La Roche Ltd
- Fujirebio Holdings, Inc.
- Genomica S.A.U.
- GlaxoSmithKline PLC
- Hologic, Inc.
- Merck & Co. Inc.
- MobileODT
- oncgnostics GmbH
- PerkinElmer, Inc.
- Qiagen N.V.
- Quest Diagnostics Incorporated
- Seegene Inc.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Zilico Ltd.
Actionable Recommendations for Industry Leaders to Accelerate Innovation, Enhance Access, and Fortify Supply Chain Resilience in Cervical Cancer Diagnostics
Industry participants must adopt a multifaceted strategy to capitalize on growth opportunities and address emerging risks. First, diversifying supply chains by qualifying alternative manufacturing sites and sourcing critical reagents from multiple geographies will minimize tariff exposure and maintain production continuity. Concurrently, investing in digital infrastructure and AI-driven analytics can accelerate assay development and optimize laboratory operations.
Second, forging public-private partnerships to support self-collection initiatives and community-based screening programs will enhance early detection rates among underserved populations. Advocacy efforts for targeted tariff exemptions on essential diagnostic equipment should be pursued through trade associations and legislative engagement, thereby reducing cost burdens for patients and providers.
Third, establishing strategic alliances with technology start-ups specializing in CRISPR-based and NGS platforms can bolster innovation pipelines and offer differentiated point-of-care testing options. Finally, embracing telehealth integration and direct-to-consumer distribution channels will expand reach, improve patient engagement, and generate real-world evidence to inform future product enhancements. By prioritizing these strategies, industry leaders can drive sustainable growth, enhance patient outcomes, and safeguard the resilience of cervical cancer diagnostic services.
Illuminating the Rigorous Research Methodology Underpinning Comprehensive Analysis, Data Triangulation, and Insight Generation in the Cervical Cancer Diagnostics Study
This study employed a rigorous, multi-phase research methodology designed to ensure the integrity and robustness of insights. Secondary research included a comprehensive review of peer-reviewed journals, industry publications, and regulatory guidelines to establish foundational knowledge and contextual understanding. Key primary sources comprised in-depth interviews with laboratory directors, clinical experts, regulatory authorities, and technology developers to capture real-time perspectives on market dynamics.
Quantitative data were validated through a triangulation process, cross-referencing proprietary hospital procurement databases, published clinical trial results, and government health statistics. Qualitative analysis leveraged thematic coding techniques to identify emerging trends, innovation drivers, and unmet clinical needs. The segmentation framework was constructed based on established classification standards, incorporating expert feedback and iterative refinement to reflect evolving laboratory practices.
To assess regional variations and tariff impacts, trade data were analyzed alongside supply chain risk reports and tariff schedules issued by government agencies. Company profiles were developed through financial filings, product launch announcements, and patent analysis, ensuring a holistic view of competitive positioning. All data points were corroborated through multiple independent sources to uphold accuracy and minimize bias. This structured approach underpins the actionable insights presented in this report.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cervical Cancer Diagnostic market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cervical Cancer Diagnostic Market, by Product Type
- Cervical Cancer Diagnostic Market, by Test Type
- Cervical Cancer Diagnostic Market, by Technology
- Cervical Cancer Diagnostic Market, by Sample Type
- Cervical Cancer Diagnostic Market, by Distribution Channel
- Cervical Cancer Diagnostic Market, by End User
- Cervical Cancer Diagnostic Market, by Region
- Cervical Cancer Diagnostic Market, by Group
- Cervical Cancer Diagnostic Market, by Country
- United States Cervical Cancer Diagnostic Market
- China Cervical Cancer Diagnostic Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1431 ]
Concluding Perspectives on the Evolution of Cervical Cancer Diagnostics Highlighting Emerging Trends, Strategic Imperatives, and the Path Forward for Stakeholders
The cervical cancer diagnostics landscape is at a pivotal juncture characterized by rapid technological evolution, shifting regulatory frameworks, and global strategic imperatives. Advanced molecular assays, AI-driven digital platforms, and patient-centric delivery models are converging to redefine how screening and early detection are conducted. However, emerging trade barriers and supply chain complexities pose tangible risks that require proactive mitigation.
By integrating robust segmentation analysis, regional market intelligence, and competitive benchmarking, stakeholders can align investments with high-impact growth areas and adapt swiftly to policy changes. Strategic collaborations, diversified sourcing, and digital innovation will be essential to maintaining market momentum and overcoming cost pressures. Ultimately, a concerted focus on accessibility, scalability, and precision will drive progress toward the shared goal of cervical cancer elimination. As the market continues to transform, the insights outlined in this report will serve as a strategic compass for decision-makers seeking to navigate the complexities of this vital healthcare segment.
Take the Next Strategic Step to Secure Comprehensive Cervical Cancer Diagnostics Insights and Connect with Ketan Rohom to Acquire the Market Research Report
If you are ready to leverage comprehensive, actionable insights into the rapidly evolving cervical cancer diagnostics landscape, take the next strategic step today. By partnering directly with Ketan Rohom, Associate Director of Sales & Marketing, you will secure immediate access to the definitive market research report that addresses transformative shifts, tariff impacts, segmentation and regional dynamics, and competitive strategies. Unlock the full suite of data, expert analysis, and personalized guidance needed to inform critical decisions, drive innovation, and strengthen your market positioning. Contact Ketan Rohom to initiate your purchase and ensure your organization remains at the forefront of cervical cancer diagnostic advancements.

- How big is the Cervical Cancer Diagnostic Market?
- What is the Cervical Cancer Diagnostic Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




