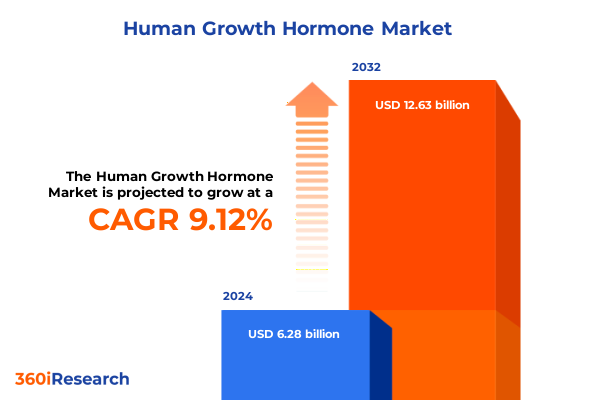
The Human Growth Hormone Market size was estimated at USD 6.75 billion in 2025 and expected to reach USD 7.25 billion in 2026, at a CAGR of 9.36% to reach USD 12.63 billion by 2032.
Unveiling the Multifaceted Role of Human Growth Hormone in Therapeutic Innovations Market Evolution and Strategic Perspectives in a Comprehensive Foundational Overview
Human growth hormone, a polypeptide produced by the anterior pituitary gland, has evolved from a rare therapeutic option to a cornerstone of diverse treatment regimens across endocrinology and rare disease profiles. Initially extracted through cadaveric sources and limited by supply constraints, the advent of recombinant DNA technology in the mid-1980s revolutionized the availability and safety profile of this critical biologic. As a consequence, growth hormone therapies expanded rapidly from pediatric growth disorders to numerous approved adult indications, setting the stage for an increasingly complex market environment.
In recent years, dynamics within the industry have shifted dramatically. Advances in bioprocessing have reduced manufacturing costs, while regulatory agencies worldwide have refined pathways to accelerate biosimilar approvals. Meanwhile, patient expectations around convenience and digital integration have risen sharply, driven by innovations such as connected delivery devices and telemedicine support models. Simultaneously, heightened scrutiny around off-label uses for anti-aging, athletic performance, and bodybuilding has prompted stakeholders to reassess marketing, compliance, and educational strategies.
Taken together, these developments underscore the urgency for pharmaceutical executives, investors, and healthcare providers to gain a nuanced understanding of the factors shaping demand, competitive positioning, and risk management in the human growth hormone sector. This introduction lays the groundwork for a deeper exploration of transformative trends, tariff impacts, segmentation insights, and actionable recommendations that will guide strategic decisions and foster sustainable growth.
Capturing the Paradigm Shift in the Human Growth Hormone Landscape Driven by Biosimilars Digital Therapeutics and Evolving Patient Engagement Models
The landscape of human growth hormone has undergone a profound transformation driven by the rapid emergence of biosimilars, the integration of digital therapeutics, and evolving paradigms of patient engagement. In recent years, regulatory bodies such as the U.S. Food and Drug Administration and the European Medicines Agency have established clear frameworks for demonstrating biosimilarity and interchangeability, enabling market entry of cost-effective alternatives to long-standing originator products. This shift has not only intensified pricing competition but also catalyzed investment in next-generation delivery systems, such as connected pens and smart cartridges, which enhance adherence and enable real-time patient monitoring.
Moreover, digital health solutions are reshaping patient interactions and care pathways. Telehealth platforms now support virtual endocrinology consultations, while mobile applications facilitate dose tracking and symptom reporting. These innovations, combined with patient support programs that emphasize self-administration in home care settings, have reduced the time to treatment initiation and improved overall health outcomes. As the industry continues to pivot toward value-based care models, partnerships among pharmaceutical companies, technology providers, and healthcare payers are forging new revenue streams and service offerings that transcend traditional drug delivery. This multi-faceted evolution underscores the critical importance of strategic adaptability for stakeholders seeking to thrive amid ongoing market disruptions.
Assessing the Strategic Implications of United States Tariffs Enacted in 2025 on the Human Growth Hormone Supply Chain Cost Structures and Competitive Dynamics
In 2025, the United States implemented a series of targeted tariffs on imported peptide and protein therapeutics, including human growth hormone sourced from key international suppliers. These measures, aimed at bolstering domestic manufacturing and protecting intellectual property, have materially increased the landed cost of originator products imported from European and Asian facilities. Consequently, pharmaceutical companies are reevaluating their supply chain footprints, accelerating investment in stateside biologics production, and renegotiating procurement agreements to mitigate margin erosion.
The tariffs’ ripple effects extend beyond cost pressures to influence competitive dynamics. Biosimilar developers with domestic production capacity have leveraged this policy shift to secure preferential pricing arrangements with payers and providers, effectively creating a two-tiered marketplace. At the same time, originator manufacturers have explored vertical integration strategies, including partnerships with contract manufacturing organizations within the U.S., to maintain market share. Longer term, ongoing dialogue between industry and policymakers will determine whether these measures persist, evolve, or give way to new trade agreements, underscoring the need for continuous monitoring of regulatory and trade landscapes.
Deriving Critical Insights from Market Segmentation Based on Type Dosage Form Application and End User to Illuminate Growth Drivers and Patient Needs
Insights derived from market segmentation illuminate the distinct drivers shaping human growth hormone deployment across product categories and patient cohorts. When considering type, originator formulations continue to benefit from established brand recognition and robust safety data, whereas biosimilars are gaining traction on the strength of lower price points and, in some cases, streamlined regulatory pathways. The evolving patent landscape has enabled multiple manufacturers to introduce biosimilar candidates, fostering competition that can ultimately improve access to therapy for cost-sensitive health systems.
Examining dosage form segmentation reveals a clear preference for patient-centric delivery mechanisms. Prefilled pens have emerged as the fastest-growing category, offering ease of use, dose accuracy, and compatibility with digital tracking tools. Cartridges, while slightly less convenient than integrated pen devices, remain popular among specialty clinics and home care providers that favor customizable dosing. Traditional vials continue to serve specific hospital settings where manual preparation and intravenous administration are standard practices, particularly in acute care scenarios.
Application-based insights further highlight where growth hormone therapies are most critical. Among approved indications, chronic kidney disease and growth hormone deficiency maintain stable demand, while rare disorders such as Prader-Willi syndrome and Turner syndrome drive specialist clinic utilization. Small for gestational age remains a niche but important category, with patient advocacy groups increasingly influencing reimbursement pathways. Off-label uses for anti-aging, athletic performance, and bodybuilding persist in informal markets, emphasizing the need for clearer educational outreach and compliance monitoring to safeguard against misuse and reputational risks.
Analysis by end user underlines the diversity of treatment settings. Home care environments have expanded significantly, supported by telehealth and direct-to-patient distribution channels. Hospitals remain vital for initial dosing, titration, and monitoring in complex cases, whereas specialist clinics leverage clinical expertise to manage rare disease protocols. Understanding these end-user preferences is critical for manufacturers seeking to tailor service offerings, optimize channel strategies, and enhance support programs that align with each care setting’s unique operational requirements.
This comprehensive research report categorizes the Human Growth Hormone market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Type
- Dosage Form
- Application
- End User
Uncovering Regional Dynamics Shaping Human Growth Hormone Adoption and Development Trends Across the Americas Europe Middle East and Africa and Asia Pacific Markets
Regional dynamics significantly influence human growth hormone adoption, with the Americas leading in both innovation and patient access initiatives. In the United States, well-developed healthcare infrastructure and favorable reimbursement frameworks have propelled widespread use of advanced delivery devices and biosimilar introductions. Concurrently, Latin American markets, while more price sensitive, are increasingly adopting originator and biosimilar therapies through government-sponsored health programs, creating pockets of rapid growth in key urban centers.
Across Europe, Middle East and Africa, regulatory alignment under the European Medicines Agency has fostered a competitive biosimilars landscape, driving down treatment costs and facilitating broader patient coverage. Nations such as Germany, France and the U.K. have implemented tenders and reference pricing, prompting manufacturers to differentiate through value-added services, including patient education and digital adherence platforms. In emerging markets within the Middle East and Africa, limited healthcare budgets and supply chain challenges hinder large-scale adoption, although targeted programs in Gulf Cooperation Council states demonstrate potential for specialized growth initiatives supported by public-private partnerships.
In the Asia Pacific region, demographic trends and government-led healthcare reforms are catalyzing demand growth. China’s domestic biotechnology sector has accelerated biosimilar development, backed by supportive policies that expedite clinical trial approvals. Japan and South Korea continue to emphasize premium originator therapies, buoyed by strong intellectual property protections and high per-capita healthcare expenditure. Meanwhile, Southeast Asian markets show increasing interest in cost-effective biosimilars, although logistical hurdles and variable regulatory environments necessitate region-specific market entry strategies. Overall, the Asia Pacific region’s heterogeneity requires nuanced approaches to pricing, distribution and stakeholder engagement to capture emerging opportunities.
This comprehensive research report examines key regions that drive the evolution of the Human Growth Hormone market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Examining Leading Company Strategies Innovation Portfolios and Collaboration Models Among Key Players Driving Competition in the Human Growth Hormone Sector
Leading companies in the human growth hormone market are deploying multifaceted strategies to sustain growth amid intensifying competition. Originator manufacturers such as Pfizer, Eli Lilly and Novo Nordisk continue to invest in lifecycle extensions through indications expansion and device innovation. For example, next-generation pen injectors with Bluetooth connectivity and dose-optimization algorithms exemplify how these firms leverage proprietary technology to maintain differentiation and elevate barriers to entry.
Conversely, biosimilar pioneers including Sandoz, Teva and LG Chem are capitalizing on cost advantages and streamlined biosimilarity pathways to gain rapid formulary access. Their strategic alliances with contract manufacturing organizations and academic research centers facilitate efficient scale-up and localized production, particularly in markets sensitive to price and supply stability. Collaboration models are also evolving: co-development agreements between originator and biosimilar developers aim to broaden patient access while sharing risks and costs associated with new clinical studies.
In parallel, technology vendors and service providers are emerging as critical partners. Companies specializing in digital dosing platforms, patient engagement software and telehealth integration are forging alliances with manufacturers to deliver holistic solutions that transcend the traditional boundaries of drug development. This convergence of life sciences and digital health underscores a broader industry imperative: to deliver therapies not only safely and effectively, but also conveniently and in ways that measurably enhance patient outcomes.
This comprehensive research report delivers an in-depth overview of the principal market players in the Human Growth Hormone market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Biopartners GmbH
- Eli Lilly and Company
- Ferring Pharmaceuticals S.A.
- Genetech Inc.
- Ipsen S.A.
- LG Chem, Ltd.
- Merck KGaA
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Roche Holding AG
- Sandoz International GmbH
- Teva Pharmaceutical Industries Ltd.
Formulating Actionable Strategic Recommendations for Industry Leaders to Optimize Biosimilar Development Diversify Supply Chains and Enhance Patient Engagement in a Competitive Market
Industry leaders should prioritize the expansion of biosimilar portfolios through targeted R&D investments and strategic partnerships that accelerate time-to-market. By securing interoperability with existing delivery ecosystems and forging alliances with technology providers, companies can enhance the patient experience and build loyalty in a price competitive environment. In parallel, organizations ought to diversify supply chain networks by investing in domestic manufacturing capacity, exploring contract manufacturing partnerships, and implementing dual sourcing strategies to hedge against tariff-driven cost volatility.
To drive sustainable growth, stakeholder engagement must extend beyond product innovation to encompass comprehensive patient support models. This involves integrating telehealth services, remote monitoring and digital adherence tools that empower providers and patients alike. Moreover, industry stakeholders should collaborate proactively with payers and regulators to design value-based pricing frameworks that align reimbursement with real-world outcomes, thereby mitigating pricing pressures and fostering shared accountability for therapeutic success.
Finally, geographic expansion strategies must be calibrated to the unique regulatory and healthcare infrastructure characteristics of each region. Tailored market entry plans, informed by local epidemiology and stakeholder mapping, will enable companies to optimize resource allocation and accelerate uptake. By combining a patient-centric mindset with robust commercial execution, industry leaders can translate insights into measurable market advantages and deliver long-term value across diverse therapeutic and geographic landscapes.
Detailing the Robust Multi Method Research Approach Combining Primary Expert Interviews Secondary Data Analysis and Expert Validation to Ensure Accuracy and Reliability
This research leveraged a rigorous, multi-method approach to ensure a comprehensive and accurate portrayal of the human growth hormone market. Primary research activities included in-depth interviews with leading endocrinologists, pediatric specialists, and hospital pharmacy directors, providing first-hand perspectives on treatment patterns, unmet needs, and device preferences. Secondary sources encompassed peer-reviewed journals, clinical trial registries, regulatory filings, and company disclosures to validate emerging trends and historical benchmarks.
Quantitative data points were triangulated through proprietary databases and public health agency statistics to establish a robust foundation for segmentation analysis and regional mapping. Patent literature and pipeline databases were reviewed to assess innovation trajectories, while intellectual property filings were systematically examined to track exclusivity timelines. This blend of qualitative insights and quantitative rigor was further enhanced by expert panel validation, in which industry veterans and thought leaders assessed preliminary findings and provided critical feedback, refining the report’s conclusions and recommendations.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Human Growth Hormone market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Human Growth Hormone Market, by Type
- Human Growth Hormone Market, by Dosage Form
- Human Growth Hormone Market, by Application
- Human Growth Hormone Market, by End User
- Human Growth Hormone Market, by Region
- Human Growth Hormone Market, by Group
- Human Growth Hormone Market, by Country
- United States Human Growth Hormone Market
- China Human Growth Hormone Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1113 ]
Concluding Reflections on the Human Growth Hormone Market Evolution Highlighting Key Insights Strategic Imperatives and Future Directions for Stakeholders to Navigate Complexity
The human growth hormone market stands at a critical juncture, shaped by technological innovation, evolving regulatory frameworks, and dynamic competitive forces. From the rise of biosimilars and digital therapeutics to the impact of U.S. tariffs and regional market nuances, stakeholders must navigate a complex array of drivers to sustain growth and enhance patient outcomes. Segmentation analyses reveal clear opportunities for targeted strategies based on product type, dosage form, indication, and care setting, while regional insights underscore the importance of tailored market entry and distribution models.
Key company case studies demonstrate that success hinges on collaborative models, device innovation, and the ability to translate clinical advantages into tangible value for payers and providers. Actionable recommendations emphasize the need to align commercial execution with patient-centric service offerings, to diversify manufacturing footprints, and to engage proactively with regulatory and reimbursement stakeholders. Collectively, these insights provide a strategic blueprint for organizations seeking to secure competitive advantages and drive long-term value.
As the market evolves, continuous monitoring of regulatory updates, supply chain developments, and emerging digital health trends will be essential. By adhering to a disciplined, data-driven approach and maintaining a patient-focused ethos, industry stakeholders can confidently navigate uncertainty and capitalize on the vast potential of human growth hormone therapies.
Engaging with Our Associate Director of Sales and Marketing to Secure Comprehensive Human Growth Hormone Market Insights and Advance Your Strategic Decision Making Today
Engaging with Ketan Rohom, Associate Director of Sales & Marketing, offers a direct pathway to accessing a detailed and authoritative analysis of the human growth hormone arena. By partnering with him, stakeholders can gain immediate clarification on specific market trends, customized data requests, and executive-level summaries tailored to organizational priorities. This personalized engagement ensures that executives, investors, and product development teams have the insights necessary to navigate regulatory complexities, supply chain challenges, and evolving competitive landscapes.
Beyond standard deliverables, Ketan Rohom can facilitate bespoke workshops, one-on-one consultations, and priority access to future updates as the industry evolves. Engaging at this level empowers companies to translate insights into actionable strategies, whether refining biosimilar launch plans, optimizing manufacturing footprints, or advancing patient engagement technologies. For decision-makers ready to accelerate growth, secure supply continuity, and capitalize on emerging therapeutic indications, contacting Ketan provides the direct support needed to transform high-level insights into measurable outcomes.
Take the next step toward data-driven decision-making and competitive differentiation in the human growth hormone market. Reach out to Ketan Rohom today to discuss report licensing options, volume discounts, and extended consulting packages. Your organization’s strategic roadmap deserves the clarity and direction that only a comprehensive, expertly curated research report can deliver. Reach out now to ensure you lead rather than follow in this dynamic therapeutic space

- How big is the Human Growth Hormone Market?
- What is the Human Growth Hormone Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




