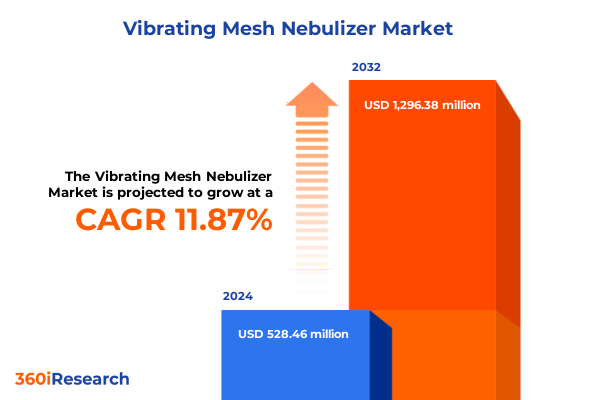
The Vibrating Mesh Nebulizer Market size was estimated at USD 592.56 million in 2025 and expected to reach USD 664.53 million in 2026, at a CAGR of 11.83% to reach USD 1,296.37 million by 2032.
Revolutionizing Respiratory Therapy through Innovative Vibrating Mesh Nebulizers Enabling Precise Aerosol Delivery and Improved Clinical Outcomes
The management of respiratory conditions has undergone a profound evolution with the advent of vibrating mesh nebulizer technology. These devices have emerged as a cornerstone in aerosol therapy, delivering precise, consistent droplet sizes that penetrate deep into the pulmonary system. Clinicians and patients alike recognize the advantages of mesh-generated aerosols, which optimize drug deposition while minimizing residual volume. As traditional jet and ultrasonic nebulizers face limitations in efficiency and noise levels, vibrating mesh systems have gained prominence for their ability to streamline treatment sessions and enhance patient adherence.
At the heart of this innovation lies a finely engineered mesh membrane that vibrates at ultrasonic frequencies, transforming liquid medications into ultra-fine mists with exceptional uniformity. The piezoelectric drivers powering these meshes operate with high energy efficiency, enabling quieter operation and extended battery life in portable models. Consequently, patients benefit from more convenient dosing regimes and greater mobility, fostering continuity of care beyond clinical settings. With these attributes, vibrating mesh nebulizers are poised to redefine respiratory therapy standards and set a new benchmark for device performance and patient experience.
Emerging Technological and Clinical Paradigm Shifts Reshaping the Vibrating Mesh Nebulizer Ecosystem for the Future of Aerosolized Drug Administration
Recent years have witnessed transformative shifts in how vibrating mesh nebulizers are designed, regulated, and adopted in clinical practice. Manufacturers have prioritized portability and user-centric designs, resulting in devices that are smaller, lighter, and quieter. This evolution satisfies the growing demand for discreet, wearable solutions that support continuous therapy, particularly for children and elderly patients managing chronic conditions. Alongside form factor improvements, the integration of smart connectivity features has accelerated; mesh nebulizers now link to mobile applications via Bluetooth and Wi-Fi to record dosage adherence and transmit treatment data to healthcare teams in real time.
Moreover, the landscape of respiratory care is being reshaped by the convergence of telemedicine platforms and digital health initiatives. Remote patient monitoring solutions leverage mesh nebulizer data to inform treatment adjustments, reducing hospital readmissions and enhancing quality of care. As regulatory bodies in key markets update guidelines to accommodate connected medical devices, stakeholders are reorienting investment towards cybersecurity, interoperability standards, and user-friendly interfaces. These paradigm shifts are driving both incremental improvements and radical innovation, positioning vibrating mesh nebulizers as pivotal instruments in the future of respiratory therapy.
Analyzing the Compounded Effects of 2025 U.S. Tariff Adjustments on Supply Chain Dynamics and Cost Structures in the Vibrating Mesh Nebulizer Industry
The 2025 revision of U.S. tariff schedules introduced additional duties on imported medical device subassemblies, including critical meshes and electronic control modules used in vibrating mesh nebulizers. By increasing the landed cost of precision components, these measures have placed upward pressure on manufacturing budgets and disrupted established sourcing strategies. In response, original equipment manufacturers are reevaluating supply-chain configurations and engaging contract partners within the Americas to mitigate tariff exposure while preserving quality and delivery timelines.
Downstream, increased procurement expenses have influenced pricing negotiations with healthcare providers and home care agencies. With reimbursement frameworks calibrated to legacy cost structures, stakeholders are conducting rigorous cost-benefit appraisals to validate the incremental value of advanced mesh solutions. Some hospital networks have deferred non-critical capital purchases, opting to extend the service life of existing fleets rather than invest in higher-priced models. Simultaneously, manufacturers are lobbying for expanded tariff exemptions on essential medical supplies and exploring nearshoring strategies to stabilize supply and safeguard profit margins.
Comprehensive Segmentation Analysis Reveals How Indication, Product Type, End User, and Distribution Channels Shape the Vibrating Mesh Nebulizer Landscape
A nuanced understanding of market segments reveals how therapeutic indications, product typologies, end-user settings, and distribution pathways converge to shape device adoption. In terms of clinical applications, asthma treatment continues to anchor demand, with patients and providers valuing the rapid onset of action and reduced treatment time that mesh nebulizers offer. Meanwhile, therapy for chronic bronchitis and COPD is driving interest in devices optimized for high-viscosity formulations, and specialized solutions for cystic fibrosis are emerging to accommodate frequent antibiotic nebulization.
Delineating product types, the dichotomy between portable and tabletop models underscores varying use cases. Portable mesh systems, whether battery operated or mains powered, are favored by home care and ambulatory populations prioritizing convenience and mobility. In contrast, pneumatic and ultrasonic mesh hybrid tabletop platforms deliver robust performance for clinical environments requiring high treatment throughput. End-user channels further influence design priorities: clinics and hospitals often emphasize device durability and service support, whereas home healthcare settings prioritize intuitive interfaces and low maintenance.
Distribution mechanisms also play a pivotal role. Traditional brick-and-mortar pharmacies remain essential for point-of-care relationships, yet online pharmacies-through e-commerce platforms and direct manufacturer portals-are rapidly gaining traction by offering subscription models, auto-replenishment services, and telepharmacy consultations. This diversification of access channels is broadening the reach of vibrating mesh nebulizers and catalyzing new engagement strategies.
This comprehensive research report categorizes the Vibrating Mesh Nebulizer market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Indication
- Product Type
- End User
- Distribution Channel
Regional Dynamics Unveiled Highlighting Differing Adoption Patterns and Growth Drivers for Vibrating Mesh Nebulizers Across the Americas EMEA and Asia-Pacific
Regional dynamics reveal distinct trajectories for vibrating mesh nebulizer adoption across the Americas, Europe Middle East & Africa (EMEA), and Asia-Pacific. In the Americas, established healthcare infrastructure and favorable reimbursement policies have fostered early uptake of advanced mesh technologies. Centers of excellence in respiratory care serve as innovation hubs, where real-world evidence studies underscore clinical benefits and inform guideline updates, further reinforcing device adoption.
Across EMEA, diverse regulatory frameworks and varying healthcare funding models create a complex operating landscape. Western European markets are characterized by rigorous certification processes under the Medical Device Regulation, ensuring high safety and performance benchmarks but extending time-to-market. Conversely, emerging markets within the region are prioritizing cost-effective solutions, driving interest in local manufacturing partnerships to tailor devices to budget constraints and patient demographics.
In the Asia-Pacific region, rapid urbanization and expanding home healthcare networks are fueling demand for portable nebulizers. Governments are increasingly investing in chronic disease management programs, while telehealth initiatives gain momentum in both metropolitan and remote areas. These factors converge to position Asia-Pacific as a strategic growth arena, where manufacturers are establishing regional hubs and customizing service models to meet diverse patient needs.
This comprehensive research report examines key regions that drive the evolution of the Vibrating Mesh Nebulizer market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Competitive Intelligence Spotlight Reveals Strategic Positioning and Innovation Pathways among Leading Vibrating Mesh Nebulizer Manufacturers
A competitive intelligence lens highlights how leading manufacturers are differentiating through innovation, strategic partnerships, and intellectual property investments. Omron Corporation leverages its proprietary vibrating mesh technology to deliver devices with ultra-fine particle distribution and robust connectivity features. The company’s collaborations with telehealth platform providers underscore its commitment to integrated care pathways and remote monitoring capabilities. Aerogen, renowned for its hospital-grade aerosol delivery systems, continues to refine its mesh geometries and invested sensor arrays to ensure consistent performance in critical care applications.
PARI GmbH has distinguished itself with modular product architectures that facilitate rapid customization for specific indications, including cystic fibrosis protocols requiring high antibiotic delivery efficiency. Meanwhile, Philips Respironics expands its ecosystem through cloud-based patient management systems, enabling clinicians to adjust treatment regimens based on real-time inhalation analytics. Smaller niche players focus on regional partnerships and targeted clinical segments, leveraging agility to co-develop specialized devices and service bundles with healthcare providers.
This comprehensive research report delivers an in-depth overview of the principal market players in the Vibrating Mesh Nebulizer market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Aerogen Limited
- DeVilbiss Healthcare LLC
- Drägerwerk AG & Co. KGaA
- Medline Industries, LP
- Nipro Corporation
- Omron Healthcare Co., Ltd.
- PARI GmbH
- Philips Respironics International S.A.
- Terumo Corporation
- Vectura Group Ltd
- Vyaire Medical, Inc.
Strategic Playbook for Manufacturers and Stakeholders to Navigate Market Complexities and Capitalize on Opportunities in the Vibrating Mesh Nebulizer Sector
Industry leaders seeking to extend their foothold in the vibrating mesh nebulizer arena must prioritize a multifaceted strategy. First, cultivating resilient supply chains through nearshoring and multi-regional sourcing will mitigate tariff and logistics risks. Partnering with local component fabricators and securing preferential trade agreements can stabilize input costs, while flexible manufacturing networks support rapid volume adjustments.
Second, integrating digital health capabilities-such as cloud-connected adherence tracking and telemedicine interoperability-will enhance value propositions for payers and providers. Investing in robust cybersecurity protocols and compliance with emerging interoperability standards will build trust and streamline regulatory clearance processes. Third, deepening clinical engagement through evidence generation and post-market surveillance initiatives will substantiate therapeutic benefits and inform reimbursement dialogues.
Finally, embracing customer-centric distribution models-ranging from subscription-based home delivery to embedded e-pharmacy consult services-will expand market access and foster long-term relationships. By orchestrating cross-functional teams that bridge R&D, regulatory, clinical affairs, and commercial operations, manufacturers can chart a course for sustained growth in a dynamic respiratory care market.
Robust Research Framework and Methodological Rigor Underpinning the Comprehensive Analysis of the Vibrating Mesh Nebulizer Market
The research underpinning this executive summary employed a rigorous, mixed-method approach to deliver actionable insights. Initially, a comprehensive secondary review of regulatory filings, peer-reviewed journals, and patent databases established a baseline understanding of technological advancements and competitive landscapes. This phase was complemented by systematic analysis of tariff schedules, trade policy documents, and supply chain reports to quantify the impact of 2025 U.S. import duties.
Subsequently, primary research entailed in-depth interviews with key opinion leaders, including pulmonologists, respiratory therapists, procurement directors, and regulatory specialists. These dialogues provided context on clinical preferences, procurement challenges, and emerging adoption criteria. Data triangulation methods synthesized secondary and primary inputs, ensuring consistency across thematic areas such as segmentation dynamics, regional nuances, and innovation trajectories.
To validate findings, an expert review panel comprising industry consultants and academic researchers evaluated the draft insights for accuracy and relevance. Ultimately, the methodology’s combination of qualitative depth and quantitative rigor yields a holistic perspective, empowering stakeholders to make informed strategic decisions in the vibrating mesh nebulizer domain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Vibrating Mesh Nebulizer market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Vibrating Mesh Nebulizer Market, by Indication
- Vibrating Mesh Nebulizer Market, by Product Type
- Vibrating Mesh Nebulizer Market, by End User
- Vibrating Mesh Nebulizer Market, by Distribution Channel
- Vibrating Mesh Nebulizer Market, by Region
- Vibrating Mesh Nebulizer Market, by Group
- Vibrating Mesh Nebulizer Market, by Country
- United States Vibrating Mesh Nebulizer Market
- China Vibrating Mesh Nebulizer Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1272 ]
Concluding Reflections on the Future Trajectory of Vibrating Mesh Nebulizer Technology Informing Strategic Decisions in Respiratory Care
In reflecting on the trajectory of vibrating mesh nebulizer technology, several key themes emerge. Technological innovation continues to drive enhancements in drug delivery efficiency, portability, and digital integration, positioning mesh devices as the preferred modality for aerosol therapy. The evolving regulatory landscape and shifting trade policies underscore the necessity of supply-chain adaptability and strategic nearshoring initiatives to sustain competitive margins.
Moreover, the interplay between market segments-spanning clinical indications, product typologies, end-user preferences, and distribution channels-highlights the importance of tailored value propositions. Successful players will be those that balance performance benchmarks with usability, cost management, and patient engagement strategies. Regional market dynamics further emphasize the need for flexible regulatory navigation and localized partnerships that address distinct healthcare infrastructure realities.
As the respiratory care environment becomes increasingly data-driven, the capacity to integrate real-time monitoring, telehealth interfaces, and evidence-based clinical validation will differentiate industry leaders. Collectively, these insights chart a path toward sustainable growth and innovation, guiding stakeholders in harnessing the full potential of vibrating mesh nebulizer technology.
Secure Your Advantage by Partnering with Ketan Rohom for Exclusive Access to the Definitive Vibrating Mesh Nebulizer Market Intelligence Report
Ketan Rohom, Associate Director of Sales & Marketing, invites you to secure a comprehensive market intelligence report that delivers unparalleled insight into the vibrating mesh nebulizer landscape. By partnering directly, you will gain access to expertly curated analysis, detailed segmentation findings, and strategic recommendations tailored to your organization’s needs. This exclusive report empowers decision-makers with the clarity to optimize product portfolios, refine supply chains, and accelerate innovation initiatives. Contact Ketan today to discuss your objectives and arrange a personalized walkthrough of key findings. Elevate your competitive positioning by obtaining the definitive resource for respiratory care market intelligence.

- How big is the Vibrating Mesh Nebulizer Market?
- What is the Vibrating Mesh Nebulizer Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




