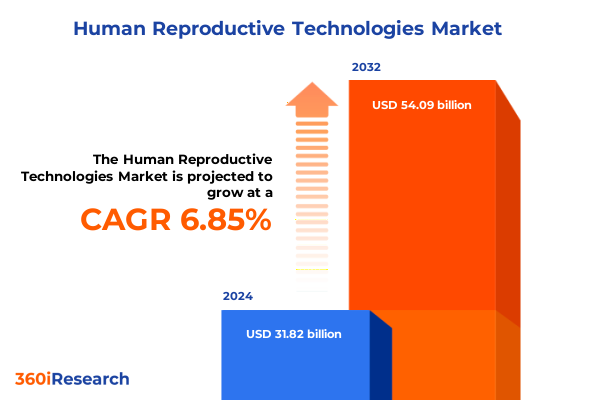
The Human Reproductive Technologies Market size was estimated at USD 32.45 billion in 2025 and expected to reach USD 34.53 billion in 2026, at a CAGR of 6.61% to reach USD 50.81 billion by 2032.
Pioneering Innovations and Patient-Centric Approaches Are Reshaping the Future of Human Reproductive Technologies Across Multiple Frontiers
The field of human reproductive technologies has undergone a profound evolution, propelled by breakthroughs in biology, automation, and personalized care models. What was once limited by rudimentary procedures and empirical protocols now thrives on precision interventions and data driven decision making. Driven by a confluence of patient expectations, technological innovation, and shifting demographic imperatives, this sector has become a cornerstone of modern healthcare. From early trial phase techniques to today’s sophisticated embryo profiling platforms, the relentless push for improved success rates and reduced invasiveness has reshaped every aspect of clinical practice.
As today’s stakeholders seek to balance efficacy with accessibility, the industry has responded with integrated solutions that span laboratory automation, digital patient engagement, and advanced diagnostic tools. Regulatory landscapes have adapted in parallel, establishing frameworks that nurture innovation while safeguarding ethical standards. Meanwhile, investment flows-from venture capital backing novel start ups to strategic partnerships among established life science leaders-are providing the financial backbone to accelerate research and commercialization. This introductory overview sets the stage for a detailed examination of the transformative forces, market segments, regional nuances, and actionable strategies that define the current era of human reproductive technologies.
Uncovering the Defining Shifts That Have Altered Clinical Practices and Technological Investments in Reproductive Medicine Over the Past Decade
Over the past decade, the reproductive health landscape has been redefined by transformative shifts in clinical pathways and technology adoption. Laboratory based processes have embraced automation and artificial intelligence to optimize embryo selection, offering unprecedented predictive accuracy and workflow efficiency. Meanwhile, digital health platforms have expanded the patient journey beyond clinical walls, enabling virtual consultations, remote monitoring, and personalized treatment planning. These changes have collectively streamlined care delivery and amplified the voice of patients in treatment decisions.
Concurrent with technological advances, evolving regulatory frameworks have both challenged and catalyzed growth. Jurisdictions across North America, Europe, and Asia Pacific have introduced adaptive pathways to ensure rigorous safety standards without stifling innovation. Intellectual property strategies have adapted to protect novel diagnostics and algorithms, fueling a competitive environment for research collaborations. In addition, shifting social attitudes and legislative changes-such as expanded coverage mandates for fertility treatments and inclusive family policies-have broadened the end user base, intensifying demand for advanced services.
Analyzing the Ripple Effects of Recent Tariff Policies on Supply Chains Equipment Accessibility and Service Delivery in Reproductive Healthcare in the US
In early 2025, the United States implemented new tariff measures targeting select medical devices and laboratory reagents, including components essential for in vitro fertilization and intracytoplasmic sperm injection protocols. As a result, manufacturers and service providers have encountered increased import costs on incubators, culture media, and specialized disposables. These elevated expenses have permeated supply chains, compelling many organizations to reexamine sourcing strategies and negotiate with a more diversified set of suppliers.
Furthermore, the tariff impacts have prompted a ripple effect on service delivery timelines. Clinics have had to adjust procurement forecasts to mitigate stock shortages, while some research institutes have delayed equipment upgrades pending clarity on tariff durations and scope. As localized manufacturing gains favor, collaborative ventures between device companies and domestic production facilities are emerging as a strategic response. However, the transition to alternative sources entails quality assurance considerations and regulatory validations, underscoring the complex balancing act between cost containment and uninterrupted patient care.
Revealing Market Response Across Assisted Reproductive Technology Modalities and Evolving Service Models While Embracing Patient Demographic Diversity
Within assisted reproductive technology modalities, the landscape encompasses procedures such as Gamete Intrafallopian Transfer and Zygote Intrafallopian Transfer alongside more prevalent approaches like In Vitro Fertilization, which itself bifurcates into fresh embryo transfer and frozen embryo transfer subprocedures. Intracytoplasmic Sperm Injection and Intrauterine Insemination continue to serve as critical pillars of clinical offerings, ensuring that treatment protocols remain tailored to patient needs and biological profiles. Transitioning to an application standpoint, the market addresses fertility preservation services including embryo cryopreservation, oocyte cryopreservation, and sperm cryopreservation, while genetic testing has advanced through preimplantation genetic testing for aneuploidy, monogenic disorders, and structural rearrangements. Infertility treatment modalities remain a foundational application, integrating next generation diagnostic assays with customized stimulation protocols.
Drug therapies play an equally vital role, with GnRH analogues orchestrating hormonal cycles and gonadotropins driving follicular development, all underpinned by human chorionic gonadotropin triggers and luteal support through progesterone. Service provisions are distributed across specialized fertility clinics, hospital based reproductive centers, and research institutes, each contributing unique capabilities to laboratory excellence, clinical care, and innovation. Finally, the end user spectrum spans couples seeking conception support-whether heterosexual or within LGBTQ communities-as well as single parents pursuing independent family building. Together, these segmentation insights reveal a nuanced ecosystem characterized by technological convergence and patient centricity.
This comprehensive research report categorizes the Human Reproductive Technologies market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Technology Type
- Fertility Drug Type
- Age Group
- Application
- End User
Examining Regional Dynamics That Drive Adoption and Spur Innovation in Reproductive Health Solutions Across the Americas Europe MEA and Asia Pacific
Regional dynamics exhibit marked variation in regulatory environments, patient demographics, and investment priorities. In the Americas, widespread insurance coverage and progressive family policies have accelerated service adoption, supporting the rollout of cutting edge lab automation and patient engagement platforms. North American clinics benefit from streamlined regulatory approvals for novel diagnostics, fostering a competitive landscape driven by consolidation and strategic alliances.
Across Europe, Middle East, and Africa, regulatory harmonization efforts are progressing unevenly. While Western European nations maintain robust reimbursement frameworks and rigorous safety evaluations, emerging economies in the Middle East are channeling sovereign wealth funds into specialized fertility centers. In parts of Africa, constrained infrastructure calls for mobile outreach programs and public private partnerships to extend basic reproductive health services. Asia Pacific markets are experiencing rapid growth fueled by demographic shifts and evolving societal norms. Countries such as Japan and South Korea emphasize advanced genetic testing, while India and China are witnessing exponential demand for fertility preservation and third party reproduction, prompting increased foreign direct investment and cross border collaborations.
This comprehensive research report examines key regions that drive the evolution of the Human Reproductive Technologies market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Trailblazing Enterprises That Are Advancing Reproductive Care Technologies Through Strategic Collaborations and Cutting Edge Research Initiatives Worldwide
Several leading enterprises are at the forefront of technological and clinical breakthroughs in reproductive care. Major life science organizations have expanded their IVF portfolios through acquisitions that integrate embryo monitoring platforms and synthetic media development. Simultaneously, specialized fertility service networks have launched partnerships with genetic testing labs and telehealth providers to deliver comprehensive patient journeys from diagnosis through treatment.
Innovative start ups are disrupting traditional models with microfluidic embryo culture systems and AI driven sperm analysis tools, often aligning with research institutes to validate novel approaches. Pharmaceutical companies are capitalizing on targeted drug delivery platforms to optimize hormonal regimens, while large equipment manufacturers continue to refine incubator designs for improved thermal stability and reduced laboratory footprints. These companies collectively underscore an ecosystem where cross sector collaboration and agile research alliances define the trajectory of next generation reproductive healthcare solutions.
This comprehensive research report delivers an in-depth overview of the principal market players in the Human Reproductive Technologies market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- FUJIFILM Holdings Corporation
- Thermo Fisher Scientific Inc
- Merck KGaA
- Bayer AG
- The Cooper Companies Inc.
- Cook Group Incorporated
- Vitrolife Group
- Progyny Inc
- Esco Lifesciences Group
- Genea Biomedx Pty Ltd
- Hamilton Thorne Inc. by Nexpring Health
- California Cryobank LLC
- Carrot Fertility, Inc.
- Cofertility Inc
- Cryoport Inc
- Ferring International Center S.A.
- Gedeon Richter Plc
- Hertility Health Ltd
- Igenomix S.L.U
- Invo Bioscience Inc
- Kindbody, Inc.
- Kitazato Corporation
- Monash IVF Group
- Natera Inc
- Organon & Co
- Overture Life Inc
- Repromed Ltd
- Virtus Health Ltd
Delivering Actionable Strategies That Empower Industry Leaders to Navigate Regulations and Capitalize on Emerging Opportunities in Reproductive Medicine
Industry leaders should prioritize supply chain resilience by cultivating relationships with diverse manufacturing partners, thereby mitigating the uncertainties introduced by changing tariff landscapes. Investing proactively in digital infrastructure, such as cloud based patient portals and remote monitoring solutions, can differentiate service offerings and enhance patient retention. Strategic alliances with genetic testing laboratories and specialized data analytics firms will enable more personalized treatment protocols and deeper insights into clinical outcomes.
Engagement with policymakers and reimbursement authorities can help shape equitable access frameworks that support long term market sustainability. Moreover, embracing outcome based contracting models and developing scalable consortiums for multi center research will facilitate faster adoption of breakthrough technologies. Finally, nurturing internal innovation pipelines through dedicated R&D incubators and talent acquisition strategies focused on bioinformatics and reproductive biology will ensure that organizations remain at the forefront of this rapidly evolving sector.
Outlining a Research Framework That Combines Quantitative Analytics with Qualitative Insights for Understanding Reproductive Technologies Ecosystem
This research combines rigorous primary interviews with fertility specialists, lab directors, and regulatory experts across multiple regions to capture real world operational insights. Secondary data sources include peer reviewed publications, clinical trial registries, and regulatory filings, which have been systematically reviewed and synthesized. Proprietary models were employed to triangulate data points, ensuring that qualitative narratives align with quantitative evidence from laboratory utilization rates and clinical outcome reports.
Expert panels convened to validate key findings and provide forward looking perspectives on emerging technologies. A structured scoring methodology assessed innovation readiness levels, market maturation, and adoption barriers across each segment. Regional analyses integrated macroeconomic indicators, trade policy reviews, and demographic statistics to portray a holistic view of market dynamics. Together, this methodological framework underpins the credibility and relevance of the study, offering stakeholders a robust foundation for strategic planning.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Human Reproductive Technologies market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Human Reproductive Technologies Market, by Technology Type
- Human Reproductive Technologies Market, by Fertility Drug Type
- Human Reproductive Technologies Market, by Age Group
- Human Reproductive Technologies Market, by Application
- Human Reproductive Technologies Market, by End User
- Human Reproductive Technologies Market, by Region
- Human Reproductive Technologies Market, by Group
- Human Reproductive Technologies Market, by Country
- United States Human Reproductive Technologies Market
- China Human Reproductive Technologies Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1590 ]
Drawing Together Core Findings to Illuminate the Collective Progress and Remaining Challenges Facing the Future of Human Reproductive Technologies
The collective progress of the human reproductive technologies sector underscores the power of interdisciplinary collaboration, where biology, engineering, and data science converge to elevate clinical outcomes. Notable strides in embryo selection fidelity, hormonal therapy optimization, and patient engagement platforms have been realized, yet challenges remain in harmonizing regulatory standards, reducing treatment costs, and ensuring equitable access across demographic groups.
Looking ahead, the interplay between next generation genetic assays, decentralized service delivery models, and artificial intelligence promises further acceleration of success rates and patient satisfaction. Continued engagement between industry stakeholders, research institutes, and public health authorities will be critical to navigate ethical considerations and scale innovations responsibly. Ultimately, the persistent drive to expand choices and improve outcomes for all individuals seeking reproductive care will define the next chapter of this transformative industry.
Invitation to Engage with Our Associate Director to Unlock Insights and Secure Your Competitive Advantage in the Evolving Reproductive Technologies Market
Are you ready to elevate your strategic decision making and secure a leadership position in the dynamic world of reproductive technologies? Ketan Rohom, Associate Director of Sales & Marketing, invites you to engage directly to customize insights that align with your unique business objectives. Whether you are seeking a deeper understanding of regional dynamics or aiming to benchmark yourself against industry pioneers, this is your opportunity to gain unparalleled clarity and confidence. By initiating a conversation today, you will unlock tailored guidance and access exclusive data that can inform your next critical move. Reach out now to transform insights into action and drive your competitive advantage.

- How big is the Human Reproductive Technologies Market?
- What is the Human Reproductive Technologies Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




