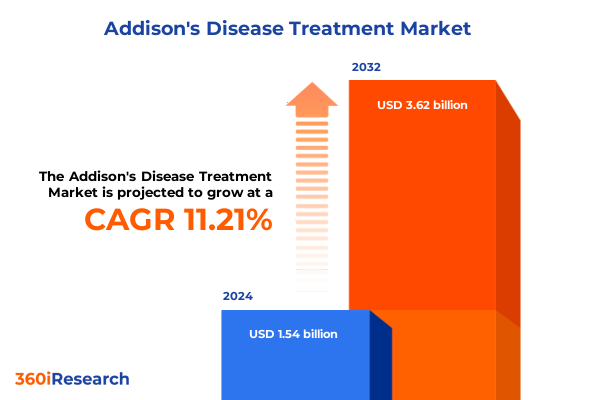
The Addison's Disease Treatment Market size was estimated at USD 1.72 billion in 2025 and expected to reach USD 1.92 billion in 2026, at a CAGR of 11.20% to reach USD 3.62 billion by 2032.
Unveiling the Complexities of the Addison’s Disease Treatment Landscape with a Holistic Overview of Therapeutic Options and Patient-Centric Imperatives
Addison’s disease, a rare endocrine disorder characterized by insufficient adrenal hormone production, presents a complex therapeutic challenge that demands nuanced clinical and commercial strategies. Patients with this condition often require lifelong management to maintain cortisol and aldosterone levels within physiological ranges, and the spectrum of available treatment modalities spans from traditional synthetic hormone replacement to cutting-edge biologics. Amid evolving regulatory landscapes and heightened patient expectations for personalized care, stakeholders must adopt a holistic understanding of both therapeutic drivers and unmet needs.
Over the past decade, therapeutic innovation has yielded refined formulations of hydrocortisone, prednisolone, and fludrocortisone, accompanied by investigational agents targeting immunological pathways implicated in adrenal insufficiency. Concurrently, heightened awareness around adrenal crisis prevention has spotlighted the importance of patient education, digital adherence tools, and streamlined delivery mechanisms. As a result, industry participants are increasingly focused on integrating clinical efficacy with real-world tolerability and patient-centric support services.
This introduction sets the stage for a strategic review of the Addison’s disease treatment market. By examining transformative shifts, policy and tariff influences, segmentation dynamics, and actionable recommendations, this report delivers a comprehensive executive summary designed to inform leaders seeking to optimize market positioning and advance patient outcomes.
Mapping the Transformative Shifts Reshaping the Addison’s Disease Treatment Ecosystem through Biotech Breakthroughs Policy Evolution and Patient-Centric Care Models
The Addison’s disease treatment ecosystem is undergoing rapid transformation driven by breakthroughs in biotechnology, precision medicine, and evolving regulatory frameworks. In recent years, several novel agents have emerged from late-stage clinical trials, promising to enhance both glucocorticoid and mineralocorticoid replacement profiles. These innovations are complemented by digital health solutions that empower patients with real-time symptom monitoring, dosage reminders, and telemedicine support.
Moreover, regulatory authorities have demonstrated increased flexibility through accelerated approval pathways and updated guidance on bioequivalence criteria for generic steroid formulations. This shift has lowered barriers to entry for biosimilars and complex generics, fostering competitive pressure and stimulating further research investment. Additionally, collaborative partnerships between pharmaceutical companies and patient advocacy organizations have elevated the importance of patient-reported outcomes in clinical development, ensuring that therapeutic innovations align closely with the lived experiences of individuals managing adrenal insufficiency.
Consequently, market participants are recalibrating their strategies to prioritize integrated care models that merge advanced pharmacotherapy with digital adherence platforms and comprehensive patient support services. These multifaceted initiatives reflect a broader industry movement toward value-based healthcare, where clinical efficacy, safety, and patient quality of life converge to define success.
Assessing the Cumulative Impact of 2025 United States Tariff Measures on the Supply Chain and Cost Dynamics of Addison’s Disease Therapies
In April 2025, the United States implemented a sweeping 10 percent global tariff on imported goods, encompassing active pharmaceutical ingredients critical to steroid replacement therapies. This measure, designed to incentivize domestic manufacturing, immediately raised procurement costs for companies reliant on foreign-sourced APIs, particularly for generic hydrocortisone and fludrocortisone. Moreover, tariffs on Chinese pharmaceutical imports spiked to as high as 245 percent, creating acute cost pressures on supply chains that source up to 40 percent of APIs from China.
Simultaneously, North American trade partners have been affected by a 25 percent levy on medical devices and raw materials originating from Canada and Mexico unless they comply with USMCA provisions. These tariffs have reverberated through the distribution networks of parenteral delivery systems and diagnostic equipment used in adrenal function testing. Additionally, a Section 232 investigation launched in April 2025 signaled the potential for further duties of up to 200 percent, underscoring ongoing policy uncertainty.
Consequently, pharmaceutical companies have accelerated efforts to onshore production and diversify supplier portfolios. Industry leaders such as AstraZeneca and Roche have announced multi-billion-dollar capital investments in U.S. manufacturing facilities to mitigate tariff exposure and safeguard supply continuity. While these strategic shifts promise long-term resilience, the near-term impact includes higher treatment costs and heightened operational complexity across the Addison’s disease therapy supply chain.
Revealing Key Market Segmentation Insights Illuminating the Diversity of Treatment Modalities Administration Routes and End-User Preferences in Addison’s Disease Therapies
A nuanced understanding of treatment type segmentation reveals the multifaceted nature of the Addison’s disease market. Biologics, encompassing ACTH analogues, monoclonal antibodies, and recombinant proteins, represent a frontier of targeted therapies aiming to modulate adrenal function with greater precision and fewer systemic side effects. In parallel, combination therapies that blend hydrocortisone with fludrocortisone or hydrocortisone with prednisone are gaining traction for their ability to address both glucocorticoid and mineralocorticoid deficiencies in a single regimen. Synthetic hormones-including fludrocortisone, hydrocortisone, and prednisone-continue to underpin standard-of-care approaches, offering established efficacy at varying cost tiers.
Administration route segmentation further delineates market dynamics. Oral formulations serve as the mainstay for chronic management, providing patient convenience, whereas parenteral options-intramuscular, intravenous, and subcutaneous-play a critical role in emergency intervention and inpatient settings. This dichotomy underscores the need for flexible distribution strategies that balance patient preferences with clinical imperatives for rapid hormone restoration.
End-user segmentation highlights the channels through which therapies reach patients, spanning ambulatory surgical centers, clinics, home healthcare providers, and hospitals. Each setting imposes unique procurement and reimbursement considerations that shape manufacturer engagement. Concurrently, distribution channels-hospital pharmacy, online pharmacy, and retail pharmacy-dictate access pathways, impacting adherence and overall treatment continuity. Finally, age group segmentation, with adult, geriatric, and pediatric subpopulations, informs tailored formulation design and dosage optimization, ensuring that therapies meet the specific physiological and lifestyle needs of diverse patient cohorts.
This comprehensive research report categorizes the Addison's Disease Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Administration Route
- End User
- Distribution Channel
- Age Group
Uncovering Critical Regional Dynamics Influencing Accessibility Affordability and Adoption of Addison’s Disease Treatments Across the Americas Europe Middle East Africa and Asia Pacific Therapeutic Markets
In the Americas, robust healthcare infrastructure and established reimbursement frameworks support widespread access to both branded and generic Addison’s disease therapies. The United States market, in particular, benefits from advanced specialty pharmacy networks and patient assistance programs that facilitate timely hormone replacement. However, disparities in rural regions underscore the importance of distribution partnerships and telehealth-enabled care pathways to bridge geographic gaps.
Europe, Middle East, and Africa present a heterogeneous landscape, where regulatory variations and differing national health policies influence treatment availability and pricing. Western European nations with centralized healthcare systems offer streamlined approval processes and negotiated pricing agreements, whereas emerging markets in the Middle East and Africa face challenges related to supply chain reliability and local manufacturing capacity. In these regions, patient advocacy initiatives play a pivotal role in raising awareness and driving policy reforms that enhance adrenal crisis preparedness.
Asia-Pacific exhibits rapid growth potential fueled by rising prevalence rates, increasing healthcare expenditure, and expanding specialty care infrastructure. Countries like Japan and Australia lead with rigorous clinical guidelines and strong local production capabilities, while Southeast Asian markets are gradually adopting advanced therapies through public-private partnerships. Meanwhile, India and China are emerging as key hubs for API manufacturing, with ongoing investments aimed at improving quality standards and scaling export volumes.
This comprehensive research report examines key regions that drive the evolution of the Addison's Disease Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Leading Company Strategies Partnerships and Pipeline Innovations Driving Competitive Dynamics in Addison’s Disease Treatment Market
Leading pharmaceutical and biotechnology companies are actively shaping the competitive landscape through strategic investments, pipeline expansions, and collaborative partnerships. Established players specializing in synthetic hormone replacement continue to optimize long-standing products with modified-release formulations and patient-centric delivery devices. At the same time, pioneering biotech firms are advancing next-generation biologics-such as monoclonal antibodies and recombinant proteins-that offer potential benefits in efficacy and safety profiles.
Several market incumbents have strengthened their footprints by forging alliances with diagnostic service providers to integrate adrenal function testing into treatment protocols, thereby enhancing early diagnosis and personalized dosing strategies. Others are exploring digital health collaborations to embed adherence tracking and teleconsultation capabilities directly into therapeutic regimens, reinforcing value propositions centered on improved clinical outcomes.
Mergers and acquisitions activity remains elevated, as companies seek to acquire complementary technologies and expand geographic reach. Meanwhile, venture capital funding for rare disease-focused startups has surged, underpinning a vibrant ecosystem of innovation aimed at addressing unmet needs in Addison’s disease management. These competitive dynamics underscore the imperative for companies to continuously innovate and differentiate their offerings in a market defined by both established standard-of-care options and emerging therapeutic frontiers.
This comprehensive research report delivers an in-depth overview of the principal market players in the Addison's Disease Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Amneal Pharmaceuticals LLC
- Bausch Health Companies Inc.
- Bayer AG
- Biogen Inc.
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Cipla Limited
- Eton Pharmaceuticals Inc.
- GlaxoSmithKline plc
- Hikma Pharmaceuticals PLC
- Lupin Pharmaceuticals
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Zydus Lifesciences
Formulating Actionable Strategic Recommendations to Empower Industry Leaders in Overcoming Challenges and Capturing Growth Opportunities in Addison’s Disease Treatment Markets
Industry leaders should prioritize strategic investment in manufacturing agility to navigate ongoing tariff uncertainties and supply chain disruptions. By diversifying supplier bases across multiple geographies and increasing capacity for in-house API production, companies can secure resilient operations and maintain consistent therapy availability.
Additionally, integrating digital health solutions with clinical offerings can elevate patient engagement and adherence. Embedding wireless monitoring tools and patient portals into treatment regimens will generate real-world evidence that supports differentiated value propositions and informs payer negotiations, reinforcing long-term market access.
Finally, forging deeper collaborations with patient advocacy groups and clinical centers of excellence will strengthen disease awareness, facilitate early diagnosis, and promote holistic care models. Such partnerships can co-develop educational resources, support adherence programs, and shape policy dialogues-ultimately positioning organizations as trusted leaders in the Addison’s disease community.
Detailing Rigorous Research Methodologies Combining Primary Expert Interviews Secondary Data Analysis and Quantitative Techniques to Ensure Comprehensive Market Intelligence
This research employs a rigorous multi-stage methodology to ensure robust insights and comprehensive market coverage. Secondary research involved an exhaustive review of peer-reviewed journals, regulatory filings, industry white papers, and public financial disclosures to map existing treatment paradigms and competitive landscapes.
Primary research comprised in-depth interviews with key stakeholders, including endocrinologists, pharmacists, hospital procurement managers, and patient advocacy leaders, to validate industry trends and capture nuanced perspectives on unmet needs. Quantitative analysis utilized a structured survey of treatment providers and payers to quantify adoption drivers, pricing dynamics, and access barriers.
Data triangulation and cross-validation techniques were applied to reconcile divergent inputs, while statistical modeling tools supported segmentation analyses. Quality assurance protocols were maintained throughout the research process, with findings subjected to expert panel reviews to confirm accuracy and relevance.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Addison's Disease Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Addison's Disease Treatment Market, by Treatment Type
- Addison's Disease Treatment Market, by Administration Route
- Addison's Disease Treatment Market, by End User
- Addison's Disease Treatment Market, by Distribution Channel
- Addison's Disease Treatment Market, by Age Group
- Addison's Disease Treatment Market, by Region
- Addison's Disease Treatment Market, by Group
- Addison's Disease Treatment Market, by Country
- United States Addison's Disease Treatment Market
- China Addison's Disease Treatment Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1590 ]
Summarizing Essential Insights and Forward-Looking Conclusions to Guide Stakeholders Through the Complex Landscape of Addison’s Disease Treatment Market
This executive summary has provided an integrated view of the Addison’s disease treatment landscape, tracing transformative shifts in therapeutic innovation, policy influences such as tariff measures, and the complex interplay of market segments, regional dynamics, and competitive forces. By weaving together insights on biologics, synthetic hormones, administration routes, and distribution channels, the report illuminates pathways to strategic differentiation.
Regional analyses underscored variations in access and affordability across the Americas, EMEA, and Asia-Pacific, while company profiles highlighted the strategic imperatives of investment in pipeline diversification, digital health integration, and stakeholder partnerships. Actionable recommendations offered targeted directions for manufacturing resilience, patient-centric care models, and policy engagement.
Collectively, these findings equip decision-makers with a nuanced understanding of current trends and provide a foundation for informed strategic planning, enabling stakeholders to capitalize on innovation, mitigate risks, and advance patient outcomes in the rapidly evolving Addison’s disease treatment market.
Seize Strategic Advantage by Connecting with Associate Director to Unlock Full Addison’s Disease Treatment Market Research Report
Are you prepared to translate these comprehensive insights into strategic action and operational excellence? Engage directly with Ketan Rohom, Associate Director, Sales & Marketing, to gain exclusive access to the full market research report and unlock tailored recommendations that will inform your next strategic move. This report offers granular data, competitive analyses, and scenario-based forecasts designed to equip your organization with the intelligence needed to lead in the rapidly evolving Addison’s disease treatment market.
Connect with Ketan Rohom today to secure your copy of the report, explore customized consulting opportunities, and ensure your strategies are backed by unmatched depth of market understanding. Elevate your decision-making process, mitigate risks, and capitalize on emerging growth areas by partnering with our expert research team.

- How big is the Addison's Disease Treatment Market?
- What is the Addison's Disease Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




