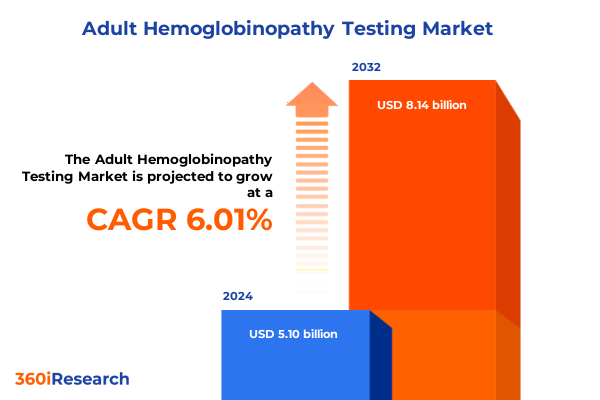
The Adult Hemoglobinopathy Testing Market size was estimated at USD 5.34 billion in 2025 and expected to reach USD 5.58 billion in 2026, at a CAGR of 6.22% to reach USD 8.14 billion by 2032.
Navigating the Evolving Landscape of Adult Hemoglobinopathy Testing with a Comprehensive Executive Overview for Informed Decision-Making
The landscape of adult hemoglobinopathy testing has evolved into a highly dynamic environment where clinical innovation intersects with patient-centric care. In this context, the executive summary serves as a strategic gateway, presenting core developments, challenges, and emerging opportunities that define the current state of diagnostic practice. At its heart lies an exploration of the convergence between laboratory precision and point of care convenience, underscoring how these divergent testing modalities collectively enhance disease detection and management. Moreover, this overview highlights the critical roles that electrophoresis, high performance liquid chromatography, genetic analysis, and rapid tests each play within the broader diagnostic framework, illustrating their complementary strengths in addressing diverse clinical scenarios.
Furthermore, understanding this domain requires recognition of how disease type considerations-from sickle cell presentations to the various thalassemia subtypes and hemoglobin C-related pathologies-shape testing priorities. By elucidating the interplay between laboratory testing platforms and point of care solutions, the report lays the groundwork for stakeholders to navigate regulatory requirements, supply chain dynamics, and patient access considerations. As such, this introduction not only sets the stage for a detailed examination of market drivers and constraints but also establishes a narrative that emphasizes the patient impact, clinical value, and technological momentum that propel adult hemoglobinopathy testing forward.
Revolutionary Technological and Clinical Advances Reshaping Adult Hemoglobinopathy Testing and Driving Enhanced Patient Outcomes Across Healthcare Systems
Recent years have witnessed a remarkable transformation in how adult hemoglobinopathies are diagnosed and monitored. Technological breakthroughs in genetic sequencing and bioinformatics have dramatically increased the throughput and accuracy of variant detection, enabling clinicians to identify even rare hemoglobin mutations with unprecedented precision. Parallel to these advances, improvements in high performance liquid chromatography and next generation electrophoresis systems have elevated the standard for routine screening, providing robust, reproducible results that inform tailored care pathways.
At the same time, the advent of portable rapid tests is reshaping access to critical diagnostics in nontraditional settings. These point of care solutions have extended screening capabilities beyond the confines of specialized laboratories, allowing for on-site confirmation of disease status in community clinics and outreach programs. This shift not only accelerates clinical decision-making but also underscores the importance of integrating centralized and decentralized testing strategies into cohesive diagnostic networks. Moreover, the proliferation of digital health platforms has facilitated seamless data exchange among laboratories, hospitals, and research institutions, unlocking new possibilities for longitudinal patient monitoring and real-world evidence generation. Together, these transformative shifts are redefining the scope of adult hemoglobinopathy testing, setting a new benchmark for personalized, patient-centric diagnostics.
Assessing the Comprehensive Consequences of Newly Implemented United States Tariffs on Testing Equipment and Reagent Supply Chains in 2025
The introduction of new tariff measures by the United States in early 2025 has exerted a profound influence on the procurement of diagnostic equipment and reagents essential to hemoglobinopathy testing. Manufacturers and distributors have faced elevated duties on imported analyzers and consumables, catalyzing shifts in sourcing strategies and cost structures throughout the supply chain. Consequently, testing laboratories have encountered increased operational expenses, prompting many to reassess vendor contracts and pursue alternative suppliers capable of delivering compliant, tariff-optimized solutions.
In response to these challenges, industry participants have explored nearshoring and domestic manufacturing initiatives to mitigate the impact of higher import costs. Collaborative partnerships between equipment producers and local reagent formulators have emerged as a key tactic for preserving supply continuity while minimizing tariff exposure. Meanwhile, end users across diagnostic laboratories, hospital networks, and research institutes have had to navigate these evolving economic conditions by adopting inventory optimization practices and renegotiating bulk procurement agreements.
Moreover, the compounded effect of tariff-related cost pressures has influenced strategic investment decisions. Point of care test developers, in particular, have accelerated efforts to localize rapid test production, ensuring that critical screening tools remain accessible in both urban centers and underserved regions. These adaptations underscore the resiliency of the adult hemoglobinopathy testing market, demonstrating a collective commitment to sustaining high-quality diagnostic services despite geopolitical and economic headwinds.
Uncovering Critical Perspectives from Modality Disease Type End User and Distribution Channel Divisions That Shape Adult Hemoglobinopathy Testing Dynamics
Insights derived from modality-based segmentation reveal that laboratory based tests continue to serve as the cornerstone of adult hemoglobinopathy diagnostics, with electrophoresis systems validating variant profiles and high performance liquid chromatography offering quantitative precision, while genetic analysis unravels underlying molecular etiologies. Parallel to this, point of care rapid tests are carving out a vital niche by delivering immediate, actionable results, particularly in decentralized screening scenarios where speed is paramount.
When examining disease type segmentation, it becomes clear that sickle cell disease remains a focal point of diagnostic innovation, driven by evolving therapeutic interventions and the need for rigorous monitoring. Thalassemia subgroups, including alpha, beta, and delta forms, demand tailored testing approaches that capture subtle shifts in hemoglobin synthesis, compelling laboratories to leverage a combination of electrophoretic separation and genetic assays for definitive classification. Hemoglobin C disease testing, though less prevalent, benefits from the same methodological rigor, ensuring that all patient cohorts receive comprehensive evaluation.
End user segmentation underscores the diverse operational demands across diagnostic laboratories, hospitals, and research institutes. Hospital laboratories, both secondary and tertiary care, emphasize rapid turnaround and integration with clinical workflows, while independent and reference laboratories focus on high-volume throughput and specialized assay offerings. Research institutions, meanwhile, prioritize flexibility in assay development to support epidemiological studies and translational research.
From a distribution channel perspective, strategic direct sales partnerships have solidified relationships between suppliers and major hospital systems, while local and regional distributors play a pivotal role in facilitating market reach across varied geographies. Online vendors complement these channels by offering streamlined procurement processes, empowering end users to source reagents and consumables with minimal lead times.
This comprehensive research report categorizes the Adult Hemoglobinopathy Testing market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Modality
- Disease Type
- Distribution Channel
- End User
Strategic Regional Variations in Laboratory and Point of Care Hemoglobinopathy Testing Across Americas EMEA and Asia Pacific Markets
Regional dynamics play a decisive role in shaping how adult hemoglobinopathy testing services are delivered and adopted. In the Americas, robust healthcare infrastructure and well-established diagnostic laboratory networks have facilitated widespread access to both conventional laboratory based tests and advanced genetic assays. Meanwhile, insurance reimbursement frameworks in this region have supported investments in high performance liquid chromatography platforms, ensuring that clinical laboratories can uphold stringent quality standards.
Within Europe, Middle East, and Africa, a heterogeneous landscape demands adaptive strategies. Western European markets benefit from cohesive regulatory alignment and substantial public funding for newborn and adult screening programs, while emerging economies in the Middle East and Africa increasingly rely on partnerships to build local testing capacity. Point of care rapid tests have become particularly impactful in remote or resource-constrained settings, bridging gaps in access and enabling earlier diagnosis of carrier status and symptomatic cases.
In the Asia-Pacific region, a combination of high population density and varied healthcare maturity levels drives significant demand for scalable testing solutions. National hemoglobinopathy screening initiatives in several countries have spurred adoption of both centralized laboratory platforms and decentralized rapid tests. Moreover, regional manufacturing hubs have emerged, supplying cost-effective equipment and reagents that serve domestic markets and fuel export opportunities. This interplay between local production and cross-border collaboration underscores the critical importance of tailored distribution strategies and regulatory navigation in fostering resilient supply chains and ensuring population-wide diagnostic coverage.
This comprehensive research report examines key regions that drive the evolution of the Adult Hemoglobinopathy Testing market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Strategic Collaborators Shaping the Future of Adult Hemoglobinopathy Diagnostics and Testing Solutions
A cohort of leading diagnostic companies has established a commanding presence in the adult hemoglobinopathy testing sphere through targeted investments in technology and strategic alliances. For instance, firms specializing in high performance liquid chromatography have expanded their portfolios to include integrated genetic testing modules, enabling comprehensive genotype and phenotype correlation within a unified workflow. Concurrently, manufacturers of electrophoresis equipment have pursued collaborations with bioinformatics providers to enhance data interpretation and reporting capabilities, supporting more nuanced clinical decision-making.
Meanwhile, several point of care test developers have accelerated their product pipelines by forging partnerships with reagent suppliers and contract manufacturers. These collaborations have yielded novel rapid test formats that deliver improved sensitivity and specificity for sickle cell and thalassemia screening. Within hospital networks, long-standing alliances with diagnostic instrument vendors have been reinforced through service agreements that encompass equipment maintenance, software upgrades, and training programs, preserving uptime and ensuring consistent testing quality.
Research institutions and diagnostic laboratories have also engaged in co-development projects with commercial entities, focusing on next generation sequencing assays that promise to uncover rare hemoglobin variants. These joint efforts highlight the value of shared expertise in driving innovation while mitigating development risk. Collectively, the strategic maneuvers of these companies illustrate an ecosystem characterized by convergence of advanced analytics, platform integration, and collaborative commercialization models, all aimed at delivering superior diagnostic solutions.
This comprehensive research report delivers an in-depth overview of the principal market players in the Adult Hemoglobinopathy Testing market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- ARKRAY, Inc.
- Beckman Coulter, Inc. by Danaher Corporation
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Bluebird Bio, Inc.
- Creative Diagnostics
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- HORIBA Group
- Illumina, Inc.
- Laboratory Corporation of America Holdings
- Merck KGaA
- Ortho Clinical Diagnostics Inc.
- PerkinElmer, Inc.
- Quest Diagnostics Incorporated
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Shimadzu Corporation
- Siemens Healthineers AG
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Tosoh Corporation
- Trinity Biotech PLC
Actionable Strategies for Industry Leaders to Capitalize on Emerging Opportunities Strengthen Supply Chains and Advance Patient-Centric Diagnostics
Industry leaders should prioritize the diversification of supply chains by forging partnerships with domestic manufacturers and local distributors to counteract tariff-related cost pressures. Embracing a balanced portfolio that integrates both centralized laboratory and point of care testing modalities will ensure resilience and adaptability in the face of evolving clinical demands. Furthermore, investing in digital infrastructure that links testing data across diagnostic laboratories, hospital systems, and research networks can unlock real-time insights, streamline workflow efficiency, and facilitate longitudinal patient monitoring.
In addition, stakeholders are advised to deepen engagement with regulatory agencies to anticipate upcoming policy changes and secure accelerated approvals for novel rapid tests and genetic assays. By fostering collaborative relationships with patient advocacy groups and clinical key opinion leaders, organizations can also shape disease management guidelines and drive broader adoption of advanced diagnostic protocols. Expanding educational initiatives aimed at healthcare providers will further reinforce the clinical utility of emerging testing technologies, supporting evidence-based integration into standard care pathways.
Moreover, exploring strategic acquisitions or joint ventures that enhance assay development capabilities and geographic reach can offer a competitive edge. Emphasizing sustainability and cost-effectiveness in reagent and consumable design will appeal to budget-conscious institutions, while maintaining stringent performance standards. Through these targeted actions, industry players can capitalize on growth opportunities, mitigate external risks, and deliver greater value to clinicians and patients alike.
Elucidating the Rigorous Multi-Source Research Methodology Employed to Generate Actionable Insights in Adult Hemoglobinopathy Testing Analysis
This analysis draws upon a comprehensive, multi-source research framework designed to deliver robust and actionable insights. Primary research efforts included in-depth interviews with hematologists, laboratory directors, and procurement specialists across multiple regions, ensuring a firsthand understanding of clinical workflows, purchasing criteria, and emerging needs. These qualitative insights were complemented by extensive secondary research, featuring a rigorous review of scientific literature, regulatory filings, company disclosures, and public health reports.
Quantitative data points were consolidated through the examination of industry databases, trade association publications, and patent filings, providing visibility into technology adoption trends and innovation pipelines. The synthesis of these data sets was facilitated by advanced analytics techniques, including thematic mapping and cross-sectional comparisons across segmentation variables and geographic markets. Peer review by an advisory panel of subject matter experts further validated key findings and interpretative frameworks, ensuring methodological transparency and analytical rigor.
To enhance reproducibility, standardized data curation protocols were applied throughout the research process, encompassing data integrity checks and bias mitigation strategies. The resulting report articulates clear linkages between research inputs and strategic conclusions, equipping stakeholders with confidence in the validity and relevance of the insights presented.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Adult Hemoglobinopathy Testing market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Adult Hemoglobinopathy Testing Market, by Modality
- Adult Hemoglobinopathy Testing Market, by Disease Type
- Adult Hemoglobinopathy Testing Market, by Distribution Channel
- Adult Hemoglobinopathy Testing Market, by End User
- Adult Hemoglobinopathy Testing Market, by Region
- Adult Hemoglobinopathy Testing Market, by Group
- Adult Hemoglobinopathy Testing Market, by Country
- United States Adult Hemoglobinopathy Testing Market
- China Adult Hemoglobinopathy Testing Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1590 ]
Synthesizing Core Findings and Future Considerations to Empower Stakeholders Driving Advancements in Adult Hemoglobinopathy Testing Ecosystems
In summary, the adult hemoglobinopathy testing landscape is characterized by rapid technological evolution, shifting economic dynamics, and diversified user requirements. Cutting-edge advances in electrophoresis, high performance liquid chromatography, and genetic analysis are converging with the proliferation of point of care rapid tests, creating a comprehensive diagnostic continuum that meets the needs of both centralized laboratories and decentralized clinical settings. The ripple effects of United States tariffs in 2025 have underscored the necessity of supply chain agility and domestic manufacturing partnerships, while regional variations highlight the importance of market-specific strategies in the Americas, Europe, Middle East, Africa, and Asia-Pacific.
Segmentation analysis reveals that a nuanced approach-tailoring assay selection to modality, disease subtype, end user profile, and distribution channel-can unlock operational efficiencies and foster deeper market penetration. The strategic activities of leading companies demonstrate an ecosystem driven by collaborative innovation, with joint ventures and co-development agreements advancing the frontiers of diagnostic accuracy. Moving forward, stakeholders are advised to adopt integrated testing models, prioritize regulatory engagement, and invest in digital platforms that enhance data interoperability and patient outcomes.
Ultimately, this executive summary serves as a foundation for informed decision-making, equipping industry leaders with the insights necessary to navigate complexities, seize emerging opportunities, and uphold the highest standards of adult hemoglobinopathy diagnostics.
Engage with Ketan Rohom Today to Discuss Tailored Insights and Secure Your Comprehensive Adult Hemoglobinopathy Testing Market Analysis Report Purchase
I appreciate your interest in deepening your strategic understanding of the adult hemoglobinopathy testing arena. To explore this comprehensive market research report tailored to your organization’s needs, please connect directly with Ketan Rohom, Associate Director of Sales & Marketing. He brings extensive expertise in translating clinical insights into actionable intelligence for decision makers. By engaging with him, you can discuss customized data deliverables, pricing structures, and access to ongoing updates. Your conversation will ensure a seamless alignment of the report’s scope with your specific research objectives, whether you require granular segmentation analysis, tariff impact evaluation, or regional benchmarking. Act now to secure this indispensable resource and reinforce your organization’s position at the forefront of adult hemoglobinopathy diagnostics and testing innovations.

- How big is the Adult Hemoglobinopathy Testing Market?
- What is the Adult Hemoglobinopathy Testing Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




