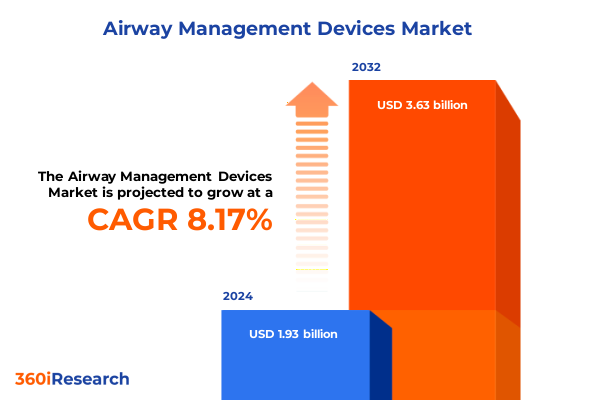
The Airway Management Devices Market size was estimated at USD 2.09 billion in 2025 and expected to reach USD 2.25 billion in 2026, at a CAGR of 8.19% to reach USD 3.63 billion by 2032.
Charting the Evolution of Airway Management Devices Through Advanced Innovation Integration and Clinical Excellence Across Healthcare Settings
Airway management devices have evolved from rudimentary instruments to sophisticated tools that are integral to patient safety, clinical efficiency, and healthcare innovation. As frontline devices deployed across operating rooms, intensive care units, and emergency settings, they facilitate critical interventions that can mean the difference between life and death. Historically centered on manual techniques and reusable components, the landscape has undergone a profound transformation driven by technological breakthroughs, evolving clinical protocols, and an imperative to reduce infection risks. In this context, stakeholders across the healthcare spectrum-including clinicians, device manufacturers, and supply chain partners-are seeking clarity on the forces shaping near-term opportunities and long-term trajectories.
This executive summary synthesizes key findings from rigorous primary and secondary research, charting the current state of the airway management device market while elucidating emerging trends. From the rise of video laryngoscopy systems that enhance visualization and first-pass success rates to the growing preference for disposable stylets and laryngeal masks designed to curtail cross-contamination, innovation remains at the forefront. At the same time, regulatory pathways, reimbursement pressures, and shifts in clinical guidelines continue to redefine procurement and adoption dynamics. By unpacking these multifaceted drivers, this report aims to equip decision-makers with a nuanced understanding of how to leverage technological, economic, and regulatory inflection points to optimize device portfolios, streamline procurement, and ultimately elevate patient outcomes.
Navigating Paradigm Shifts in Airway Management Device Landscape Driven by Technological Advancements and Clinical Demands
The airway management device landscape is experiencing transformative shifts fueled by a confluence of technological advancements, clinical insights, and operational demands. Video laryngoscopy has rapidly emerged from a niche solution to a mainstream modality, offering enhanced glottic visualization that translates into higher success rates on initial intubation attempts. This paradigm shift is complemented by the integration of robotic-assisted intubation platforms in select academic centers, signaling a future where automation and artificial intelligence may further augment clinician performance and reduce procedure times.
Alongside digital enhancements, material science breakthroughs are reshaping device ergonomics and safety profiles. The adoption of biocompatible silicone formulations for laryngeal masks and endotracheal tubes is reducing hypersensitivity reactions and improving patient comfort during extended procedures. Concurrently, antimicrobial coatings on stylets and laryngoscope blades are gaining traction, as hospitals intensify efforts to curtail device-associated infections. These innovations coincide with the proliferation of telemedicine-enabled airway training simulators, allowing practitioners to hone skills remotely and ensuring consistency in clinical competencies across geographic regions.
Operationally, the rapid growth of ambulatory surgical centers and home care settings is driving demand for compact, user-friendly airway management solutions. Devices that balance portability with performance are increasingly prioritized, reflecting a broader healthcare shift toward decentralized care delivery. Ultimately, these transformative shifts underscore the imperative for manufacturers, healthcare providers, and policymakers to collaborate on harmonizing innovation with affordability, sustainability, and clinician training to realize the full potential of next-generation airway management technologies.
Assessing the Cumulative Impact of 2025 United States Tariffs on Airway Management Device Supply Chains and Market Dynamics
The introduction of new tariff measures by the United States in 2025 has created significant ripples across the airway management device supply chain. Raw materials and finished components sourced from key international manufacturing hubs now incur additional duties, elevating input costs for device producers. This has prompted several leading manufacturers to reassess their procurement strategies, shifting a portion of component production to domestic or nearshore sites in order to mitigate exposure to unpredictable trade policies. While these adjustments have led to incremental increases in unit manufacturing costs, they also underscore a strategic pivot toward supply chain resilience.
Moreover, distributors and hospital group purchasing organizations have begun renegotiating contract terms to reflect the evolving cost structure. In some instances, long-standing volume-based rebate agreements have been recalibrated to incorporate tariff pass-through clauses, transferring a degree of commercial risk back to suppliers. Despite temporary margin compression, this realignment is fostering greater transparency around total landed costs and reinforcing the importance of collaborative forecasting. Looking ahead, sustained dialogue between policymakers, manufacturers, and healthcare providers will be critical to balancing national trade objectives with the imperative of ensuring uninterrupted access to lifesaving airway management devices.
Unearthing Critical Insights Across Diverse Segmentation Dimensions Including Product Type Material Selection Insertion Technique End User Application and Size
Diverse segmentation dimensions collectively illuminate the nuanced drivers of demand and innovation in the airway management device sphere. When examining product types-including endobronchial tubes, endotracheal tubes, intubation stylets, laryngeal mask airways, and laryngoscopes-it becomes clear that each category presents its own set of clinical priorities. Within endotracheal tubes, the distinction between cuffed and uncuffed designs caters to varying patient profiles and procedural protocols, influencing procurement preferences in pediatric versus adult care settings.
Material considerations are equally pivotal. Polyvinyl chloride remains widely utilized for its cost efficiency and flexibility, whereas silicone variants command a premium for superior biocompatibility and reduced risk of mucosal irritation. Insertion techniques further delineate market segments, with direct laryngoscopy retaining relevance in resource-limited contexts and video laryngoscopy gaining preference in high-acuity environments due to its enhanced visualization capabilities. End users span ambulatory surgical centers that prize compact footprint devices, emergency medical services requiring ruggedized portable kits, home care settings focused on ease of use, and hospitals demanding high-throughput performance.
Applications across emergency care, home care, intensive care, operating rooms, and pre-hospital scenarios underscore the versatile role of airway devices throughout the patient journey. Within each context, device attributes such as sterility assurance, ergonomic design, and compatibility with ancillary equipment drive selection criteria. Finally, size-based segmentation-encompassing adult, neonatal, and pediatric categories-ensures that device dimensions and mechanical properties align with the anatomical and physiological requirements of distinct patient cohorts. Synthesizing these segmentation insights reveals critical inflection points for targeted product development, pricing strategies, and clinical training programs.
This comprehensive research report categorizes the Airway Management Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Material
- Insertion Technique
- End User
- Application
- Size
Revealing Regional Dynamics Shaping the Airway Management Device Market Across the Americas Europe Middle East Africa and Asia Pacific
Regional dynamics exert a profound influence on both the trajectory of innovation and the pace of adoption for airway management devices. In the Americas, robust healthcare infrastructure coupled with high procedural volumes in operating rooms and intensive care units drives demand for advanced video laryngoscopy systems and disposable device variants. This region also benefits from well-established reimbursement frameworks that support capital investment in cutting-edge airway management technologies.
In Europe, Middle East & Africa, heterogeneous healthcare delivery models shape a bifurcated landscape. Western European markets exhibit strong uptake of premium devices underpinned by centralized purchasing consortia, while emerging economies across the Middle East and Africa prioritize cost-effective solutions with simplified training requirements. Meanwhile, regulatory harmonization efforts such as the European Medical Device Regulation have elevated quality standards, fostering a competitive environment where innovation must align with stringent compliance measures.
The Asia-Pacific region is characterized by rapid expansion of ambulatory surgical centers and growing investments in emergency medical services infrastructure. Local manufacturers are increasingly focusing on region-specific design enhancements and price-sensitive product portfolios to capture market share. At the same time, collaborations between multinational corporations and domestic partners are accelerating technology transfer and localized production, thereby enhancing accessibility and reducing lead times. Taken together, these regional nuances underscore the need for strategic go-to-market frameworks that account for regulatory complexity, clinical preferences, and economic variability across global markets.
This comprehensive research report examines key regions that drive the evolution of the Airway Management Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Strategic Competitive Landscape and Key Company Initiatives Driving Innovation Growth and Collaboration in the Airway Management Devices Sector
The competitive landscape in the airway management device sector is defined by a blend of established medical device conglomerates and specialized innovators. Large-scale manufacturers leverage their expansive distribution networks and broad product portfolios to cross-sell complementary devices and consumables, thus reinforcing their market positions. These incumbents are increasingly channeling R&D investments toward smart-enabled laryngoscopes that integrate sensor-based feedback and software analytics, aiming to differentiate on data-driven clinical decision support.
Concurrently, niche players are carving out focused segments by pursuing disruptive material science breakthroughs and user-centric design philosophies. Some startups are pioneering single-use, bioresorbable stylets that eliminate reprocessing burdens while minimizing environmental footprint. Others have introduced portable video laryngoscopy attachments compatible with off-the-shelf tablets and smartphones, democratizing access to advanced visualization in resource-limited settings. Partnerships between these agile companies and academic institutions or healthcare systems facilitate clinical validation studies that underpin market credibility and accelerate adoption.
Strategic collaborations and targeted acquisitions are further shaping competitive dynamics. Established firms often acquire or invest in early-stage innovators to access proprietary technologies and digital capabilities. These alliances not only expand product roadmaps but also foster integrated solutions that span the continuum of airway management-from simulation-based training modules to remote monitoring of procedural outcomes. Collectively, these competitive maneuvers underscore a marketplace in which agility, cross-disciplinary collaboration, and a relentless focus on clinical value are paramount.
This comprehensive research report delivers an in-depth overview of the principal market players in the Airway Management Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Ambu A/S
- Armstrong Medical Ltd.
- Becton, Dickinson and Company (BD)
- ConvaTec Group Plc
- Cook Medical Incorporated
- Fisher & Paykel Healthcare
- Flexicare (Group) Limited
- ICU Medical
- Intersurgical Ltd.
- KARL STORZ SE & Co. KG
- Medline Industries, LP
- Medtronic plc
- Mercury Medical
- Olympus Corporation
- SunMed
- Teleflex Incorporated
- Tracoe Medical GmbH
- VBM Medizintechnik GmbH
- Verathon Inc.
- Vyaire Medical
Formulating Actionable Recommendations to Empower Industry Leaders in Driving Sustainable Growth Innovation and Regulatory Compliance in Airway Management Devices
To thrive in this evolving environment, industry leaders must embrace multidimensional strategies that synergize innovation with operational excellence. First, forging collaborative partnerships with clinical centers of excellence will accelerate product validation and foster clinician advocacy, facilitating smoother market entry. By co-developing training curricula and simulation programs, device manufacturers can ensure that end users derive maximum value from advanced features and maintain procedural proficiency.
Second, adopting a modular product architecture can drive cost efficiencies while enabling rapid customization. By designing core platforms with interchangeable components, companies can respond nimbly to diverse clinical requirements, size specifications, and budget constraints. This approach also supports localized manufacturing strategies, which can mitigate the impact of geopolitical trade policies and enhance supply chain resilience.
Third, leveraging digital health integrations-such as cloud-based analytics and AI-driven performance feedback-will differentiate offerings and create new revenue streams. Manufacturers should explore software-as-a-medical-device (SaMD) frameworks to deliver real-time insights, facilitate remote troubleshooting, and generate outcome-based evidence that informs value-based procurement discussions.
Finally, proactive engagement in regulatory affairs and reimbursement policy dialogues is critical. By participating in standards development organizations and contributing to health economic assessments, companies can influence the evolution of guidelines and secure favorable coverage pathways. Collectively, these recommendations will empower industry leaders to drive sustainable growth, maintain competitive differentiation, and deliver enhanced patient care through next-generation airway management solutions.
Outlining Rigorous Research Methodology Incorporating Comprehensive Data Collection Qualitative and Quantitative Analyses and Expert Validation for Robust Findings
This study integrates a robust research methodology to ensure the highest level of accuracy and credibility. Initially, an extensive secondary research phase was conducted, encompassing peer-reviewed medical literature, regulatory filings, public company disclosures, and clinical guidelines to map the competitive, technological, and regulatory contours of the airway management device landscape. These insights established a comprehensive baseline for subsequent primary research.
During the primary research phase, in-depth interviews were held with senior executives from device manufacturers, procurement specialists at major hospital networks, and key opinion leaders in anesthesiology and emergency medicine. These qualitative discussions elucidated nuanced perspectives on innovation priorities, adoption barriers, and procurement dynamics. Additionally, targeted surveys gathered structured feedback from frontline clinicians across diverse geographic regions, capturing preferences related to device ergonomics, material properties, and digital integrations.
Quantitative analysis was performed using a proprietary database that tracks device registrations, product launches, and clinical adoption rates. Cross-validation techniques compared survey responses against real-world procurement trends and regulatory approval timelines to enhance data integrity. Finally, an expert advisory panel comprising industry veterans and academic researchers reviewed draft findings, providing critical inputs that refined the final deliverables. Through this multi-pronged methodology, the study delivers a rigorous and actionable intelligence framework for stakeholders across the airway management device ecosystem.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Airway Management Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Airway Management Devices Market, by Product Type
- Airway Management Devices Market, by Material
- Airway Management Devices Market, by Insertion Technique
- Airway Management Devices Market, by End User
- Airway Management Devices Market, by Application
- Airway Management Devices Market, by Size
- Airway Management Devices Market, by Region
- Airway Management Devices Market, by Group
- Airway Management Devices Market, by Country
- United States Airway Management Devices Market
- China Airway Management Devices Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1272 ]
Drawing Conclusive Perspectives on the Future Trajectory of Airway Management Devices Amidst Technological Evolution Regulatory Shifts and Clinical Challenges
As the healthcare sector continues to emphasize patient safety, operational efficiency, and cost containment, airway management devices will remain a pivotal focal point for innovation and strategic investment. The convergence of advanced visualization technologies, biocompatible materials, and digital analytics heralds a new era of precision in airway interventions. At the same time, supply chain resilience and responsive manufacturing frameworks have become non-negotiable prerequisites in the wake of shifting trade policies and global disruptions.
Moving forward, stakeholders that prioritize collaborative co-creation with clinical partners, embrace modular and scalable product architectures, and integrate data-driven capabilities will be best positioned to capture value in the dynamic airway management market. Furthermore, regional nuances in regulatory environments, reimbursement frameworks, and healthcare infrastructure will continue to shape adoption patterns, underscoring the importance of localized strategies. Ultimately, the intersection of clinical efficacy, economic sustainability, and regulatory foresight will define the trajectory of this critical segment, guiding manufacturers and healthcare providers toward delivering superior outcomes and sustained competitive advantage.
Seize Strategic Insights Today By Connecting with Ketan Rohom to Propel Your Airway Management Innovations Forward
Embark on the journey toward harnessing unparalleled market intelligence by engaging with Ketan Rohom, the Associate Director of Sales & Marketing. His expert guidance will ensure you gain immediate access to the full breadth of insights and actionable strategies delineated in this comprehensive report. By initiating a dialogue with Ketan, you can seamlessly align your innovation pipeline, sales channels, and marketing initiatives with the dynamic contours of the airway management device market. Connect today to secure the competitive edge that your organization deserves and transform research insights into tangible business growth.

- How big is the Airway Management Devices Market?
- What is the Airway Management Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




