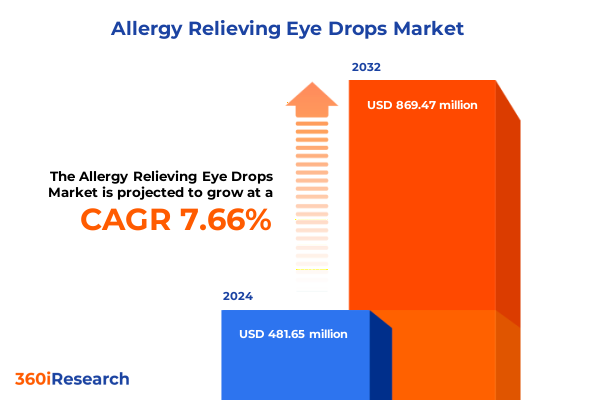
The Allergy Relieving Eye Drops Market size was estimated at USD 519.37 million in 2025 and expected to reach USD 552.20 million in 2026, at a CAGR of 7.63% to reach USD 869.47 million by 2032.
Understanding the Allergy Relieving Eye Drops Market: A Comprehensive Introduction to Patient Needs, Treatment Paradigms, and Emerging Growth Drivers
Allergic conjunctivitis, commonly referred to as an eye allergy, represents a significant global health concern driven by environmental factors such as pollen, dust mites, mold, and animal dander. In the United States, recent epidemiological data indicate that between 15 percent and 25 percent of the population-approximately 50 to 85 million Americans-experience ocular allergy symptoms at some point, affecting productivity, comfort, and quality of life. These symptoms, characterized by itching, redness, tearing, and swelling of the conjunctiva, arise when the immune system overreacts to external allergens, triggering histamine release that compromises ocular comfort and visual performance.
Eye drops remain the cornerstone of allergy management, offering targeted relief through antihistamines, mast cell stabilizers, and combination therapies that address immediate and delayed inflammatory responses. Patients increasingly seek solutions that deliver rapid symptom control while minimizing irritation, leading to wider adoption of both over-the-counter and prescription formulations. Simultaneously, growing consumer awareness and advancements in digital health platforms are reshaping how these treatments are prescribed and accessed. As a result, industry stakeholders must navigate evolving patient expectations, regulatory landscapes, and competitive dynamics to capitalize on the expanding demand for effective, convenient, and safe ocular allergy treatments.
Exploring Transformative Shifts Reshaping the Allergy Eye Drop Landscape: From Digital Health Adoption to Innovative Formulations Driving Market Evolution
The allergy eye drop market is undergoing transformative shifts driven by innovations in drug formulation, digital healthcare integration, and evolving consumer behaviors. Dual-action agents that combine antihistamines with mast cell stabilizers are gaining traction, offering patients longer-lasting relief and improved adherence by reducing the frequency of dosing. This focus on combination therapies underscores a broader industry trend toward patient-centric design, where efficacy and convenience are both prioritized to enhance treatment compliance and outcomes.
Concurrently, the rise of e-commerce and telehealth has revolutionized distribution and prescription channels. Online pharmacies and company-owned websites now serve as primary points of sale for allergy eye drops, propelled by the pandemic’s lingering impact on consumer preferences for home delivery and remote consultation. Pharmaceutical companies are ramping up digital marketing campaigns, leveraging automated refills and personalized offers to engage tech-savvy consumers and streamline their patient journeys. Moreover, personalized medicine is emerging as a potential differentiator, with research into genetic and immunological markers paving the way for tailored therapies that address specific allergen sensitivities and patient profiles.
Analyzing the Cumulative Impact of 2025 United States Tariffs on Allergy Eye Drop Supply Chains, Production Costs, and Market Accessibility
In 2025, U.S. tariffs have introduced new complexities for allergy eye drop manufacturers and distributors, their cumulative impact reverberating throughout supply chains and cost structures. Starting April 5, a 10 percent global tariff on all imports added an extra layer of cost to active pharmaceutical ingredients, packaging materials, and finished products, compounding existing duties under Section 301 that range from 7.5 to 25 percent on Chinese and Indian imports. Additionally, the Commerce Department initiated a Section 232 investigation into pharmaceuticals on April 1, 2025, focusing on finished drug products, APIs, and critical inputs to assess national security implications; this probe has introduced uncertainty that could precipitate tariffs of up to 200 percent if restrictions are enacted.
Beyond official investigations, sector-specific tariffs have been levied to curb reliance on foreign suppliers. APIs sourced from China now face a 25 percent duty, while those from India incur a 20 percent tariff, directly inflating manufacturing costs for generic and branded allergy eye drops. Packaging materials such as glass vials and analytical instruments have been hit with 15 percent tariffs, further disrupting downstream production workflows. In response, major pharmaceutical firms are recalibrating sourcing strategies, investing in domestic manufacturing capacity, and forging strategic partnerships to mitigate cost pressures and safeguard market accessibility.
Deriving Key Segmentation Insights in the Allergy Eye Drop Market Across End Users, Distribution Channels, Ingredient Types, Applications, and Forms
Insight into end-user dynamics reveals that over-the-counter products dominate patient access, driven by the convenience of self-medication and the desire for immediate relief. However, prescription formulations maintain a critical role, especially among patients with chronic or severe ocular allergy, where tailored dosing and physician oversight ensure optimal therapeutic outcomes. The coexistence of these pathways necessitates distinct go-to-market strategies for OTC brands and prescription drug manufacturers.
Distribution channels exhibit marked stratification. Convenience stores and pharmacy outlets provide traditional touchpoints for consumer purchase, while hospital pharmacies cater to more complex clinical requirements. Online retail has emerged as a vital channel, encompassing company-owned websites that offer brand-specific promotions and third-party marketplaces that aggregate diverse product portfolios. Chain pharmacies, with their extensive reach, complement independent pharmacy stores that serve local communities, together forming a multifaceted network that meets a spectrum of patient needs.
Ingredient innovation is advancing the market through antihistamines that deliver rapid symptom relief, combination therapies that extend duration of action, decongestants that address redness, and mast cell stabilizers that prevent allergic flare-ups. The balance between efficacy and tolerability is critical, prompting manufacturers to refine formulations for improved ocular comfort.
Allergy eye drops are applied across perennial and seasonal contexts, reflecting the need for year-round management of indoor allergens like dust mites, as well as targeted relief during seasonal pollen spikes. In parallel, formulation form factors-multi-dose dispensers favored for cost-effectiveness and single-dose vials prized for sterility and convenience-shape prescribing and purchasing behaviors across diverse patient cohorts.
This comprehensive research report categorizes the Allergy Relieving Eye Drops market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Ingredient Type
- Form
- Application
- End User
- Distribution Channel
Revealing Key Regional Insights Impacting the Allergy Relieving Eye Drops Market Dynamics Across Americas, Europe, Middle East & Africa, and Asia-Pacific
In the Americas, the United States leads with strong consumer awareness, an established network of retail pharmacies, and proactive OTC regulations that facilitate easy access to allergy eye drops. The region’s sophisticated healthcare infrastructure supports rapid adoption of innovative formulations, while e-commerce penetration continues to climb, offering new avenues for market expansion. Conversely, Latin America’s growing urbanization and environmental pollutants are driving demand for accessible antihistamine solutions, though reimbursement challenges and distribution gaps remain obstacles to be addressed.
Europe, the Middle East, and Africa (EMEA) present a heterogeneous landscape. Western Europe benefits from progressive regulatory frameworks, particularly the approval of preservative-free formulations that cater to sensitive eyes and chronic users. In contrast, Eastern European markets are characterized by price sensitivity and limited access to branded therapies, fostering competition from generic and private-label products. Middle Eastern and African markets are at different stages of maturity, with demand heavily influenced by urban pollution levels and healthcare investment trends.
Asia-Pacific is experiencing the fastest growth trajectory, underpinned by rising allergy awareness, expanding pharmacy chains, and supportive government initiatives aimed at improving healthcare access. Countries such as China and India, despite imposing higher tariffs on pharmaceutical imports, are seeing domestic manufacturers scale up production of affordable eye drop formulations. Japan and Australia remain innovation hubs, with strong investments in novel delivery systems and digital health integration fueling market sophistication.
This comprehensive research report examines key regions that drive the evolution of the Allergy Relieving Eye Drops market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Evaluating Key Companies Shaping the Allergy Eye Drop Market with Strategic Partnerships, Innovative Launches, and Competitive Differentiation
Major players are deploying diverse strategies to secure market leadership. Alcon and Bausch & Lomb continue to leverage their global distribution networks and deep clinical expertise to introduce combination therapies and preservative-free eye drops that address both acute symptoms and long-term management needs. Glenmark Pharmaceuticals capitalized on the expanding OTC segment in the U.S. by launching olopatadine hydrochloride ophthalmic solution in August 2024, reflecting agile product development aligned with consumer preferences for preservative-free options.
Simultaneously, AstraZeneca has embarked on a $50 billion investment initiative in U.S. manufacturing and R&D infrastructure, a strategic response to potential high tariffs and national security-driven trade policies. This reshoring effort underscores a broader industry trend among leading pharmaceutical companies-such as Roche, Novartis, and Johnson & Johnson-to diversify supply chains, enhance domestic capacities, and hedge against trade policy volatility. Emerging regional players, particularly in Asia, are leveraging cost advantages and localized expertise to introduce competitive antihistamine and mast cell stabilizer formulations, intensifying market competition.
This comprehensive research report delivers an in-depth overview of the principal market players in the Allergy Relieving Eye Drops market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- AbbVie Inc.
- Alcon Inc.
- Alembic Pharmaceuticals Limited
- Astellas Pharma Inc.
- Bausch Health Companies Inc.
- Bayer AG
- Dr. Reddy’s Laboratories Ltd.
- Hoffmann-La Roche Ltd.
- Johnson & Johnson
- Lupin Limited
- Merck & Co., Inc.
- Nicox S.A.
- Novartis AG
- Ocular Therapeutix, Inc.
- Pfizer Inc.
- Prestige Consumer Healthcare Inc.
- ROHTO Pharmaceutical Co., Ltd.
- Rynel Clifton Pharma Pvt. Ltd.
- Sager Pharma Kft.
- Santen Pharmaceutical Co., Ltd.
- Similasan Corporation
- Starpharma Holdings Limited
- Sumitomo Pharma Co., Ltd.
- Sun Pharmaceutical Industries Limited
- Teva Pharmaceutical Industries Ltd.
Delivering Actionable Recommendations for Industry Leaders to Enhance Supply Resilience, Innovate Products, and Capitalize on Emerging Allergy Treatment Trends
Industry leaders should prioritize supply chain diversification to mitigate the impact of tariffs and ensure uninterrupted access to critical APIs. Establishing partnerships with domestic manufacturers or regional suppliers can reduce dependency on high-tariff sources and enhance resilience against policy shifts. In parallel, investing in advanced manufacturing technologies-such as continuous processing and modular fill–finish systems-can streamline production and offset rising input costs.
Product portfolios should be expanded to include dual-action formulations and preservative-free variants that cater to patient demand for both efficacy and comfort. Customizing marketing messages to emphasize these benefits, alongside leveraging digital health platforms for patient education, will strengthen brand differentiation and foster loyalty. Furthermore, integrating telehealth consultations with automated dispensing services can create seamless patient experiences and unlock new revenue streams.
Finally, companies must engage proactively with policymakers and industry associations to advocate for balanced trade policies that consider essential healthcare needs. By providing evidence-based insights on supply chain dependencies and patient impacts, stakeholders can help shape regulations that support sustainable market growth while safeguarding national security interests.
Detailing the Rigorous Research Methodology Underpinning the Allergy Eye Drops Market Report Incorporating Multisource Data and Analytical Frameworks
This report synthesizes quantitative and qualitative insights derived from a combination of primary and secondary research methodologies. Primary research encompassed in-depth interviews with ophthalmologists, allergists, industry executives, and key opinion leaders to capture real-world perspectives on prescribing behaviors, unmet needs, and emerging product preferences. Concurrently, structured surveys of end users provided granular data on consumer experiences, purchasing channels, and brand perceptions.
Secondary research involved a comprehensive review of industry publications, regulatory filings, company annual reports, and trade association data to map product pipelines, tariff developments, and regional market dynamics. Proprietary databases were leveraged to analyze pricing trends, shipment volumes, and distribution footprints. Data triangulation techniques ensured validation and consistency across sources, while statistical modeling and scenario analysis facilitated the assessment of tariff impacts and growth drivers.
All data points were subjected to rigorous quality control checks, including cross-referencing against multiple sources and expert validation, to uphold accuracy and relevance. Market segmentation frameworks were established based on end user, distribution channel, ingredient type, application, and form, enabling targeted analysis of subsegments. The result is a robust, data-driven market intelligence resource that informs strategic decision-making across the allergy eye drop value chain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Allergy Relieving Eye Drops market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Allergy Relieving Eye Drops Market, by Ingredient Type
- Allergy Relieving Eye Drops Market, by Form
- Allergy Relieving Eye Drops Market, by Application
- Allergy Relieving Eye Drops Market, by End User
- Allergy Relieving Eye Drops Market, by Distribution Channel
- Allergy Relieving Eye Drops Market, by Region
- Allergy Relieving Eye Drops Market, by Group
- Allergy Relieving Eye Drops Market, by Country
- United States Allergy Relieving Eye Drops Market
- China Allergy Relieving Eye Drops Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1113 ]
Synthesizing the Conclusion Emphasizing Critical Takeaways, Market Opportunities, and Strategic Imperatives for Allergy Relieving Eye Drops Stakeholders
The allergy relieving eye drops market is at a pivotal juncture, shaped by evolving patient expectations, technological advancements, and shifting regulatory landscapes. Transformative shifts in digital health and e-commerce, coupled with formulation innovations such as dual-action and preservative-free products, are redefining market dynamics. At the same time, U.S. tariffs and trade policy uncertainties underscore the importance of resilient supply chains and strategic manufacturing decisions.
Segmentation analysis reveals nuanced opportunities across OTC and prescription channels, diverse distribution networks, and specialized product types tailored for seasonal and perennial applications. Regionally, growth is concentrated in North America and Asia-Pacific, while EMEA markets display heterogeneous adoption patterns influenced by regulatory and economic factors. Leading companies are responding with targeted investments, strategic partnerships, and product launches that reflect agile market positioning.
As industry stakeholders navigate these complexities, informed decision-making and proactive strategy implementation will be critical. By leveraging comprehensive market insights and embracing adaptability, organizations can capitalize on emerging trends and secure competitive advantage in a rapidly evolving allergy eye drop landscape.
Compelling Call-to-Action to Connect with Ketan Rohom for Acquiring the Comprehensive Allergy Relieving Eye Drops Market Research Report
Unlock unparalleled insights and strategic foresight by securing the comprehensive Allergy Relieving Eye Drops Market Research Report today. Engage with Ketan Rohom, Associate Director of Sales & Marketing, to explore how this report can empower your organization with data-driven strategies, timely trend analysis, and actionable market intelligence. Don’t delay; contact Ketan to purchase our in-depth report and position your company at the forefront of allergy eye drop market innovation.

- How big is the Allergy Relieving Eye Drops Market?
- What is the Allergy Relieving Eye Drops Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




