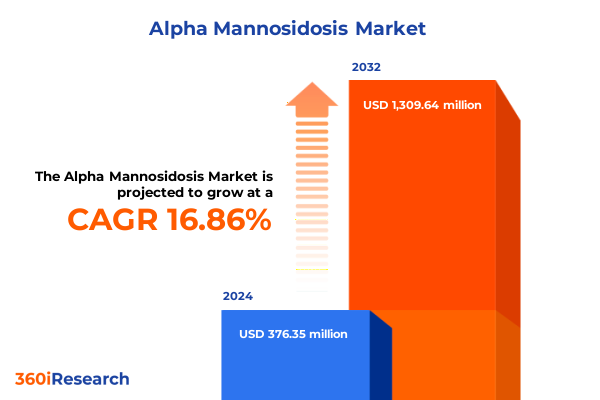
The Alpha Mannosidosis Market size was estimated at USD 432.99 million in 2025 and expected to reach USD 503.44 million in 2026, at a CAGR of 17.12% to reach USD 1,309.64 million by 2032.
Unveiling the Critical Role of Counteracting Genetic Lysosomal Accumulation and Advancing Therapies to Address Alpha Mannosidosis Challenges
Alpha mannosidosis is a rare autosomal recessive lysosomal storage disorder characterized by a deficiency of the alpha-D-mannosidase enzyme due to pathogenic variants in the MAN2B1 gene. This enzymatic deficit leads to the progressive accumulation of mannose-rich oligosaccharides within lysosomes, resulting in multisystem cellular dysfunction. Current estimates indicate that the condition affects approximately one in every 500,000 to 1,000,000 individuals globally, underscoring its classification as an orphan disease with significant unmet medical needs.
Building upon this genetic understanding, clinical manifestations span a spectrum from mild cognitive impairment and hearing loss to profound skeletal abnormalities and immune deficiencies. Patients frequently present with distinct craniofacial features, including a prominent forehead and prognathism, alongside muscle weakness and hepatosplenomegaly. Early-onset, severe variants often culminate in reduced life expectancy, whereas individuals with milder phenotypes may survive into adulthood, carrying lifelong burdens that impact quality of life and health-care resource utilization.
The diagnostic journey for alpha mannosidosis is frequently prolonged due to clinical overlap with other lysosomal storage disorders and limited awareness among health-care providers. Definitive diagnosis relies on demonstrating reduced alpha-mannosidase activity in leukocytes or fibroblasts and confirming MAN2B1 gene mutations through molecular genetic testing. As the community of researchers, clinicians, and advocacy groups unites to address these challenges, there is a growing impetus to implement newborn screening pilots, develop robust natural history studies, and accelerate therapeutic innovation across diverse stakeholder networks.
Revolutionary Regulatory Approvals and Pioneering Gene Therapy Innovations Are Reshaping the Therapeutic Landscape for Alpha Mannosidosis
The therapeutic landscape for alpha mannosidosis has been fundamentally transformed by landmark regulatory milestones that validate enzyme replacement therapy as a viable intervention. In February 2023, the U.S. Food and Drug Administration approved lamzede (velmanase alfa) as the first enzyme replacement therapy for non-central nervous system manifestations of alpha mannosidosis. This approval followed a rigorous phase III trial demonstrating significant improvements in functional endpoints such as stair-climbing and pulmonary capacity, coupled with oligosaccharide normalization over 52 weeks of treatment. Prior to this, the European Medicines Agency’s Committee for Medicinal Products for Human Use had recommended lamzede for marketing authorization under exceptional circumstances in January 2018, emphasizing the critical need for post-authorization studies to further characterize long-term safety and pediatric efficacy.
Beyond regulatory approvals, the field has seen the emergence of robust long-term clinical data that reinforce the value proposition of enzyme replacement therapy. At the 20th Annual WORLDSymposium in February 2024, Chiesi Global Rare Diseases presented outcomes from up to 12 years of continuous velmanase alfa administration, highlighting durable biochemical and clinical responses across a heterogeneous patient cohort. These findings underscore the therapy’s capacity to stabilize disease progression and foster sustained improvements in exercise tolerance and metabolic parameters.
Simultaneously, groundbreaking preclinical efforts are advancing gene therapy as a complementary modality, particularly for addressing neurological disease components unmet by systemic enzyme delivery. In feline models, a single cisterna magna infusion of adeno-associated virus serotype 1 encoding alpha-mannosidase achieved widespread neuronal transduction, delayed symptom onset, and extended survival, providing a compelling proof-of-concept for central nervous system targeting. Parallel investigations leveraging rAAV6 for choroid plexus transduction have demonstrated restoration of enzyme activity throughout the murine brain, positioning choroid plexus–directed gene transfer as a promising strategy for future clinical translation.
Together, these regulatory wins, long-term efficacy data, and innovative gene-based approaches are converging to redefine possibility within alpha mannosidosis care. The integrated momentum across approval pathways, clinical evidence generation, and translational research signals a new era of therapeutic potential for patients and stakeholders alike.
Assessing the Multifaceted Consequences of 2025 United States Tariff Policies on Treatment Access and Supply Dynamics in Alpha Mannosidosis
United States tariff initiatives enacted in 2025 have introduced a complex overlay on the supply and affordability of therapies for alpha mannosidosis. In April, a blanket 10% global tariff on imported goods was extended to include critical health-care inputs such as active pharmaceutical ingredients and biologic therapies, accompanied by elevated duties-245% on selected Chinese-sourced APIs and 25% on finished pharmaceutical products from key trading partners. These measures, aimed at bolstering domestic manufacturing capacity, have nonetheless induced cost headwinds across the pharmaceutical value chain.
A consequential analysis by Ernst & Young, commissioned by the U.S. pharmaceutical industry, revealed that a 25% tariff on pharmaceutical imports could inflate annual U.S. drug expenditures by nearly $51 billion and drive wholesale prices upward by as much as 12.9%. This impact is especially pertinent given that U.S. imports of finished pharmaceutical products totaled $203 billion in 2023, with 73% originating from European markets. Should tariffs be fully passed through, both branded and specialty therapies for rare diseases would face significant price pressures that could hinder patient access and strain payer budgets.
The ripple effects of tariff-induced cost increases extend to the domestic production ecosystem. Tariffs on imported API inputs, which constitute roughly 30% of pharmaceutical imports in 2023, are forecast to raise U.S. manufacturing expenses by 4.1%, potentially undermining the competitiveness of both generic and specialty drug producers. Generic manufacturers, operating on thin margins, may be compelled to shift additional tariffs directly onto end-users, thereby exacerbating affordability challenges for patients receiving essential therapies in hospitals and specialty clinics.
Further complicating the policy environment are concerns regarding compliance with World Trade Organization exemptions for pharmaceuticals, potential retaliatory duties, and the efficacy of proposed reshoring incentives. These uncertainties underscore the imperative for carefully designed exemptions and phased implementations to safeguard the continuity of care for individuals living with alpha mannosidosis and other rare conditions.
Delineating Therapy Type, Indication Variations, and End-User Practices to Illuminate Segmentation-Driven Opportunities in Alpha Mannosidosis Care
Based on Therapy Type, the treatment spectrum for alpha mannosidosis is charted across bone marrow transplantation and enzyme replacement therapy. Hematopoietic stem cell transplantation offers the potential for long-term enzyme supply through donor-derived cells, although its application is constrained by donor availability, procedural risks, and the necessity for early intervention. By contrast, enzyme replacement therapy provides a less invasive, standardized infusion regimen that has demonstrated consistent biochemical normalization and functional gains, making it a preferred option for many patients and clinicians.
Based on Indication Type, the clinical heterogeneity of alpha mannosidosis is stratified into Type I mild variants presenting after age ten, Type II moderate forms most often identified in early childhood, and Type III severe manifestations that emerge in infancy with rapid progression. This tripartite classification informs clinical decision-making, guiding therapeutic prioritization and tailoring surveillance protocols to the distinct trajectories associated with each subtype. Effective segmentation by indication thus enhances patient stratification and optimizes outcomes across the disease continuum.
Based on End-User, both hospitals and specialty clinics serve as critical nodes in the alpha mannosidosis care network, each navigating unique logistical and reimbursement landscapes. Hospitals frequently manage the multidisciplinary care of severe cases requiring transplantation or acute management of complications, while specialty clinics often focus on long-term follow-up, infusion administration, and comprehensive metabolic monitoring. The interplay between these care settings shapes treatment accessibility, therapy adherence, and ultimately patient quality of life across diverse health-care systems.
This comprehensive research report categorizes the Alpha Mannosidosis market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Therapy Type
- Indication Type
- Diagnosis Method
- End-User
Examining Regional Treatment Ecosystems Across Americas, Europe Middle East & Africa, and Asia-Pacific to Reveal Strategic Insights for Alpha Mannosidosis
In the Americas, particularly within the United States and Canada, a mature regulatory environment has facilitated the approval of innovative therapies such as velmanase alfa, while advanced reimbursement mechanisms-including orphan drug incentives and value-based arrangements-seek to balance cost and patient access. Nonetheless, high orphan drug prices and payer scrutiny of real-world outcomes continue to shape adoption dynamics, prompting manufacturers to demonstrate long-term value through patient registries and outcome-based contracting.
Across Europe, the Middle East & Africa, regulatory frameworks hinge on the European Commission’s centralized marketing authorization, which enabled lamzede’s approval under exceptional circumstances. Individual member states in Western Europe have since negotiated pricing and reimbursement pathways, whereas many Middle Eastern and African jurisdictions grapple with limited rare disease infrastructure and funding constraints. This regional heterogeneity underscores the need for tailored market access strategies that account for divergent health-care budgets and policy priorities.
In the Asia-Pacific region, countries such as Japan and Australia are actively evaluating both enzyme replacement and emerging gene therapies, with evolving health-technology assessment processes that emphasize cost-effectiveness and budget impact. While approvals in Japan mirror those in Western markets, reimbursement negotiations remain rigorous, and in many emerging markets within Asia-Pacific, limited awareness and diagnostic capacity pose fundamental barriers to timely treatment initiation and comprehensive care delivery.
This comprehensive research report examines key regions that drive the evolution of the Alpha Mannosidosis market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Strategic Collaborations Driving Enzyme Replacement and Gene Therapy Pipelines in the Alpha Mannosidosis Arena
Chiesi Group has solidified its leadership in enzyme replacement therapy for alpha mannosidosis through the commercialization of velmanase alfa and the presentation of extensive long-term data at global symposiums. The company’s strategic engagement at events like WORLDSymposium and its commitment to rare disease registries reflect a proactive approach to evidence generation and stakeholder collaboration.
Simultaneously, pioneering gene therapy efforts by academia and biotechnology firms are advancing rAAV-based platforms targeting the central nervous system. Preclinical successes in feline and murine models, exemplified by rAAV1 and rAAV6 delivery strategies, have catalyzed partnerships aimed at translating these approaches into early-phase human studies. Such collaborations between vector technology providers and clinical research centers are critical to bridging preclinical innovation and clinical development pathways.
At the same time, leading multinational pharmaceutical companies are forging licensing agreements with Chinese biotech firms to access novel platform technologies and expand their early-stage pipelines. This trend, which has seen a surge in cross-border collaborations valued at over $13 billion in 2025 alone, underscores the strategic imperative of diversifying innovation sources even as trade policy uncertainties loom.
Collectively, these corporate strategies illustrate a dynamic ecosystem in which established rare disease leaders, emerging biotech innovators, and global pharma-biotech alliances converge to drive sustained progress in alpha mannosidosis research and therapy development.
This comprehensive research report delivers an in-depth overview of the principal market players in the Alpha Mannosidosis market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abeona Therapeutics Inc.
- Alexion Pharmaceuticals, Inc. by AstraZeneca PLC
- Amicus Therapeutics, Inc.
- BioMarin Pharmaceutical Inc.
- CHIESI Farmaceutici S.p.A.
- Eli Lilly and Company
- JCR Pharmaceuticals Co., Ltd.
- Kamada Ltd.
- Orchard Therapeutics PLC
- Pfizer, Inc.
- Protalix Biotherapeutics
- Quest Diagnostics Incorporated
- Sanofi S.A.
- Sarepta Therapeutics, Inc.
- Takeda Pharmaceutical Company Limited
- Ultragenyx Pharmaceutical Inc.
Strategic Imperatives for Industry Leaders to Enhance Collaboration, Diversify Supply Chains, and Accelerate Patient-Centric Innovations in Alpha Mannosidosis
Industry leaders must convene cross-sector consortia that integrate academic researchers, clinical specialists, patient advocacy organizations, and commercial partners to streamline translational research efforts. Such consortia can harmonize data collection protocols, scale natural history studies, and expedite multicenter trials that evaluate both enzyme replacement and gene therapy candidates in parallel.
Comprehensive Research Methodology Integrating Peer-Reviewed Data Sources, Clinical Trial Analyses, and Expert Consultations for Alpha Mannosidosis Insights
Our research methodology encompassed a comprehensive review of peer-reviewed literature, including PubMed-indexed clinical trials and translational studies, analysis of regulatory documents from the U.S. Food and Drug Administration and the European Medicines Agency, and synthesis of company press releases and financial disclosures. We cross-referenced these secondary sources with clinical trial registries to verify study statuses and endpoints.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Alpha Mannosidosis market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Alpha Mannosidosis Market, by Therapy Type
- Alpha Mannosidosis Market, by Indication Type
- Alpha Mannosidosis Market, by Diagnosis Method
- Alpha Mannosidosis Market, by End-User
- Alpha Mannosidosis Market, by Region
- Alpha Mannosidosis Market, by Group
- Alpha Mannosidosis Market, by Country
- United States Alpha Mannosidosis Market
- China Alpha Mannosidosis Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 795 ]
Synthesizing Transformative Trends and Critical Insights to Forge a Cohesive Strategic Direction in Alpha Mannosidosis Management and Development
The field of alpha mannosidosis research and treatment is at an inflection point marked by regulatory breakthroughs, robust long-term clinical data, and transformative gene therapy prospects. While tariff pressures and regional disparities present access challenges, strategic segmentation insights, and targeted recommendations can guide stakeholders toward resilient supply chains and equitable patient access. The synthesis of diverse therapeutic modalities-from bone marrow transplantation to systemic ERT and CNS-focused gene therapy-affirms a multifaceted approach that holds the promise of meaningful clinical impact.
Empowering Decision-Makers to Secure Detailed Market Intelligence and Propel Alpha Mannosidosis Advancements with Customized Research Solutions
Take this opportunity to access the comprehensive market research report on alpha mannosidosis and gain the detailed insights you need to inform strategic decisions. Ketan Rohom, Associate Director of Sales & Marketing at 360iResearch, is available to guide you through the full suite of analyses, including regulatory landscapes, competitive profiles, and tailored recommendations. Engage with Ketan to secure your copy of the report and empower your organization to lead in the evolving alpha mannosidosis ecosystem.

- How big is the Alpha Mannosidosis Market?
- What is the Alpha Mannosidosis Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




