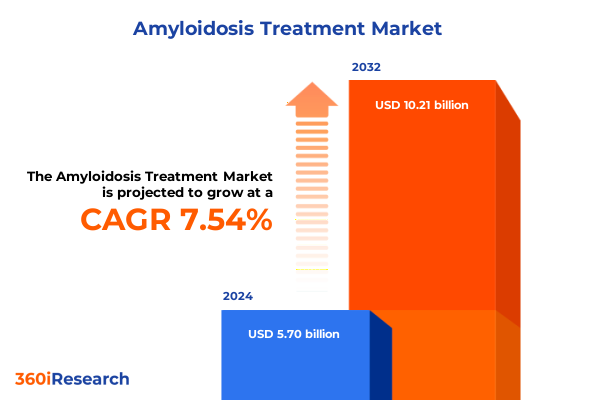
The Amyloidosis Treatment Market size was estimated at USD 6.13 billion in 2025 and expected to reach USD 6.59 billion in 2026, at a CAGR of 7.56% to reach USD 10.21 billion by 2032.
Exploring the Complex Therapeutic Landscape of Amyloidosis and Key Drivers Influencing Treatment Innovation and Adoption in the United States
Emerging therapeutic innovations and patient care imperatives have coalesced to form a complex yet opportunity-laden landscape for amyloidosis treatments in the United States. As awareness of this heterogeneous group of protein-misfolding disorders rises, the urgency to refine diagnostic pathways, enhance treatment efficacy, and broaden patient access becomes paramount. Clinicians and stakeholders are navigating a rapidly evolving environment shaped by novel drug approvals, shifting reimbursement policies, and heightened focus on personalized medicine. Consequently, a clear synthesis of current drivers, barriers, and market catalysts is essential to guide strategic decision-making.
This executive summary provides a structured overview of the amyloidosis treatment domain, beginning with foundational context on disease subtypes, diagnostic advancements, and evolving therapeutic modalities. It situates recent innovations-ranging from monoclonal antibodies to transthyretin (TTR) silencers-within broader healthcare delivery dynamics. Through this lens, the subsequent sections distill transformative shifts, the cumulative impact of national trade policies, and critical segmentation insights. By weaving together clinical, economic, and policy perspectives, this introduction sets the stage for a nuanced understanding of the current state of amyloidosis care and highlights key levers for strategic growth.
Unprecedented Scientific Breakthroughs and Regulatory Advances Redefining the Amyloidosis Therapeutic Landscape and Patient Outcomes Across the Nation
The landscape of amyloidosis treatment has been reshaped by unprecedented scientific breakthroughs and evolving regulatory frameworks. In recent years, gene silencing therapies targeting transthyretin have transitioned from preclinical promise to clinical reality, reflecting a paradigm shift in addressing hereditary ATTR amyloidosis. Alongside small interfering RNA (siRNA) modalities, the advent of next-generation monoclonal antibodies has expanded the repertoire of options for light chain (AL) amyloidosis, offering disease-modifying potential where conventional cytotoxic regimens previously dominated.
Concurrently, diagnostic advancements have played a pivotal role in accelerating early detection and patient stratification. High-sensitivity cardiac imaging and novel biomarker panels have transformed clinical practice, enabling physicians to identify organ involvement at subclinical stages. This confluence of precise diagnostics and targeted therapies has catalyzed a move away from one-size-fits-all approaches, fostering a more nuanced, subtype-specific treatment algorithm. Finally, regulatory agencies have streamlined accelerated approval pathways for breakthrough therapies while reinforcing post-marketing surveillance, ensuring that safety remains paramount even as innovation accelerates.
Assessing the Far-Reaching Economic and Supply Chain Impacts of United States Tariff Measures on Amyloidosis Treatment Accessibility by 2025
United States tariff measures enacted through 2025 have exerted broad influence on the accessibility and cost structure of amyloidosis therapies. A significant portion of active pharmaceutical ingredients (APIs) for proteasome inhibitors and monoclonal antibodies originates from international supply chains sensitive to import duties. Heightened tariff rates on certain excipients and mRNA synthesis components have incrementally raised manufacturing overhead, leading to pricing pressures that ripple from contract manufacturers to end-user institutions. These cost escalations are particularly pronounced for novel agents such as TTR silencers, which rely on sophisticated lipid nanoparticle delivery systems.
Moreover, disruptions in the global trade environment have prompted manufacturers to reassess procurement strategies and diversify supplier bases. Companies are increasingly adopting dual-sourcing models and regionalized manufacturing hubs to mitigate the risk of tariff-induced shortages. Payers and health systems have responded by renegotiating formulary agreements and exploring outcome-based contracting to manage total cost of care. Although tariffs have introduced near-term challenges, they have also stimulated a strategic pivot toward localized production and integrated supply-chain resilience, paving the way for more stable access to life-saving amyloidosis therapies.
Unraveling Critical Segmentation Insights for Treatment Modalities Routes of Administration End Users Drug Classes and Disease Variants
A critical examination of amyloidosis market segmentation reveals nuanced insights into treatment adoption, patient experience, and commercial potential. When considering treatment type, the market bifurcates into pharmacological interventions, high-dose chemotherapy followed by stem cell transplantation, and supportive therapies that address organ dysfunction. Pharmacological treatments encompass a diverse spectrum: alkylating agents such as melphalan remain foundational in AL amyloidosis, immunomodulators like lenalidomide provide synergistic efficacy, and monoclonal antibodies such as daratumumab offer targeted eradication of plasma cell clones. Proteasome inhibitors, including bortezomib, carfilzomib and ixazomib, continue to enhance response rates, while TTR silencers inotersen and patisiran represent a transformative mechanism in ATTR subtypes. Further, stabilizing agents such as acoramidis and tafamidis act by preserving native transthyretin structure to prevent fibril formation.
The route of administration significantly influences patient adherence and healthcare resource utilization, spanning intravenous infusions in hospital settings to oral regimens viable for home care and emerging subcutaneous formulations that bridge the gap between convenience and efficacy. End-user segmentation underscores the evolving role of specialty clinics in delivering complex therapies outside traditional inpatient environments, contrasted with hospital centers managing acute presentations and home care services facilitating long-term maintenance.
Drug class segmentation mirrors treatment type but provides clarity on molecular mechanisms, reinforcing the importance of alignment between therapeutic target and patient phenotype. Finally, amyloidosis type segmentation categorizes patients into AA, AL, hereditary ATTR and wild-type ATTR cohorts, each distinguished by unique pathophysiology and varying responses to the aforementioned classes of agents.
This comprehensive research report categorizes the Amyloidosis Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Route Of Administration
- Drug Class
- Amyloidosis Type
- End User
Exploring Regional Variation in Amyloidosis Treatment Adoption and Innovation Across Americas Europe Middle East Africa and Asia Pacific Markets
Geographical heterogeneity exerts a profound influence on amyloidosis treatment patterns and commercial dynamics. In the Americas, the United States leads innovation through robust clinical trial activity and rapid adoption of newly approved agents, while Canada contributes through collaborative post-market registries that refine real-world usage insights. Specialty centers in Brazil and Mexico are progressively integrating novel therapies, albeit with reimbursement challenges that temper widespread access.
In Europe, robust healthcare infrastructures and centralized procurement policies have facilitated structured rollout of next-generation treatments. Scandinavian registries, the UK National Amyloidosis Centre, and German hematology networks exemplify coordinated adoption, even as Middle Eastern and African markets grapple with fragmented access and variable reimbursement environments. Nonetheless, philanthropic partnerships and public-private initiatives are gradually bridging these gaps.
Asia-Pacific presents a mosaic of maturity levels: Japan’s early regulatory approval for key agents has expedited access, while China’s evolving biosimilar market introduces cost-effective alternatives for proteasome inhibitors. Australia’s universal healthcare model supports broad coverage of TTR stabilizers and silencers, whereas India is emerging as a manufacturing hub for generic formulations. Across this diverse region, collaborative research consortia are amplifying patient recruitment and genotype-phenotype correlation studies, driving a more comprehensive understanding of regional disease burden and treatment response.
This comprehensive research report examines key regions that drive the evolution of the Amyloidosis Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Analyzing Leading Biopharmaceutical Innovators and Strategic Collaborations Driving Amyloidosis Treatment Development and Market Dynamics
Leading biopharmaceutical companies are at the vanguard of amyloidosis innovation, driving R&D pipelines and strategic partnerships. Global leaders such as Alnylam Pharmaceuticals and Ionis Pharmaceuticals have propelled TTR silencer technology to market, leveraging proprietary RNA therapeutics platforms to achieve landmark clinical outcomes. These achievements have spurred competitive responses from established oncology and rare disease divisions within major players like Pfizer and Janssen, each exploring next-generation gene editing and antisense oligonucleotide approaches.
Collaborations between academic centers and industry have also intensified, with research institutes in the United States and Europe co-authoring pivotal trials that inform regulatory submissions. Additionally, specialized biotech firms such as Prothena and Akcea Biosciences have carved niches around monoclonal antibodies and stabilizers, engaging in licensing agreements that enhance portfolio breadth. Strategic acquisitions by larger pharmaceutical entities are further reshaping the competitive landscape, consolidating novel modalities under broader corporate umbrellas and enabling streamlined commercialization pathways. This convergence of capabilities underscores an ecosystem in which innovation, partnership, and strategic investment collectively accelerate therapeutic advancements.
This comprehensive research report delivers an in-depth overview of the principal market players in the Amyloidosis Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbvie, Inc.
- Acrotech Biopharma, Inc
- Alexion Pharmaceuticals
- Alnylam Pharmaceuticals Inc.
- Arcturus Therapeutics, Inc.
- Astellas Pharma, Inc.
- AstraZeneca PLC
- Attralus, Inc.
- BridgeBio Pharma, Inc.
- Bristol-Myers Squibb Company
- Corino Therapeutics, Inc.
- GlaxoSmithKline PLC
- Ionis Pharmaceuticals, Inc.
- Johnson & Johnson Services Inc
- Merck & Co., Inc.
- Neurimmune AG
- Oncopeptides AB
- Pfizer Inc.
- Proclara Biosciences Inc.
- Prothena Corporation PLC
- Regeneron Pharmaceuticals Inc.
- SOM INNOVATION BIOTECH, SA,
- Sorrento Therapeutics, Inc.
- Spectrum Pharmaceuticals, Inc.
- Takeda Pharmaceutical Company Limited
Formulating Strategic Recommendations for Industry Leaders to Capitalize on Emerging Opportunities in Amyloidosis Therapies and Patient Care Pathways
Industry leaders must adopt a proactive posture to capitalize on the rapidly evolving amyloidosis ecosystem. Prioritizing investment in biomarker discovery and early diagnostics remains critical, as timely intervention significantly improves patient prognoses and differentiates emerging therapies in competitive markets. Simultaneously, forging alliances with contract manufacturers and technology providers ensures supply chain resilience, mitigating tariff-related risks and safeguarding uninterrupted drug availability. It is also imperative to develop outcome-based contracting models with payers, aligning reimbursement with real-world performance metrics to demonstrate value while managing cost pressures.
Engagement strategies should extend beyond traditional channels by integrating digital health platforms that support patient monitoring and adherence. Tailored patient support programs, rooted in behavioral insights, can enhance therapy persistence and capture longitudinal data that informs iterative clinical development. Furthermore, cross-functional collaboration between commercial, medical affairs, and health economic teams can refine market access strategies, ensuring that evidence generation aligns with payer requirements and patient advocacy priorities. By focusing on these multidimensional initiatives, industry leaders will strengthen their competitive positioning and drive sustainable growth in the amyloidosis treatment arena.
Detailing Robust and Transparent Research Methodology Employed to Derive Comprehensive Insights into Global Amyloidosis Therapeutic Trends
The research methodology underpinning this analysis blends rigorous primary and secondary techniques to ensure validity and granularity. Key opinion leader interviews, encompassing hematologists, cardiologists, and pharmacoeconomists across major markets, provided firsthand insights into clinical adoption barriers and evolving treatment algorithms. These qualitative inputs were systematically triangulated with data from peer-reviewed journals, clinical trial registries, patent filings, and health authority databases to validate developmental timelines and regulatory statuses.
An extensive secondary research phase leveraged company publications, conference proceedings, and real-world evidence databases to map competitive landscapes and supply-chain dynamics. Quantitative surveys of institutional formularies and payer policy frameworks augmented this foundation, offering a comprehensive view of reimbursement environments. Data synthesis employed cross-validation techniques to reconcile discrepancies and highlight consensus trends. Finally, dedicated analytical frameworks assessed tariff implications and regional market characteristics, ensuring a robust, transparent approach that supports actionable insights and strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Amyloidosis Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Amyloidosis Treatment Market, by Treatment Type
- Amyloidosis Treatment Market, by Route Of Administration
- Amyloidosis Treatment Market, by Drug Class
- Amyloidosis Treatment Market, by Amyloidosis Type
- Amyloidosis Treatment Market, by End User
- Amyloidosis Treatment Market, by Region
- Amyloidosis Treatment Market, by Group
- Amyloidosis Treatment Market, by Country
- United States Amyloidosis Treatment Market
- China Amyloidosis Treatment Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 2067 ]
Synthesizing Key Findings on Treatment Advances Market Dynamics and Strategic Imperatives Guiding the Future of Amyloidosis Care
This executive summary articulates the convergence of scientific innovation, policy evolution, and strategic imperative that defines the future of amyloidosis care. Breakthroughs in gene silencing and monoclonal antibody therapies have redefined treatment paradigms, while advanced diagnostics and evidence-based contracting frameworks are reshaping patient access. Navigating tariff-driven supply chain shifts underscores the importance of resilience and localization in manufacturing strategies. Detailed segmentation analysis illuminates pathways for targeted commercialization by aligning therapeutic class, administration route, end-user setting, and amyloidosis subtype.
Regional insights reveal differentiated adoption curves, with mature markets showcasing rapid uptake and emerging economies poised for growth as infrastructure and reimbursement mechanisms evolve. Leading companies and strategic collaborations form the backbone of innovation, driving pipeline expansion and portfolio diversification. Ultimately, the synthesis of these findings highlights critical imperatives for industry stakeholders: invest in early diagnostics, fortify supply chains, engage payers through value-based models, and leverage digital tools to optimize patient engagement. These actions will collectively accelerate the translation of scientific advances into improved outcomes for patients with amyloidosis.
Driving Stakeholder Engagement with Ketan Rohom Insights and Opportunities Available in the Comprehensive Amyloidosis Treatment Market Research Report
To unlock unparalleled insights into therapeutic trends, market dynamics, and strategic opportunities in the amyloidosis treatment landscape, reach out to Ketan Rohom, Associate Director, Sales & Marketing, for tailored guidance. His expertise can help stakeholders align their commercial strategies with cutting-edge research findings and gain a competitive edge. By partnering with Ketan Rohom, you will navigate complex data with confidence and access exclusive add-ons that enhance decision-making for product development, market entry, and stakeholder engagement. Don’t miss this chance to secure your copy of the comprehensive market research report and accelerate your next phase of growth in amyloidosis therapies.

- How big is the Amyloidosis Treatment Market?
- What is the Amyloidosis Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




