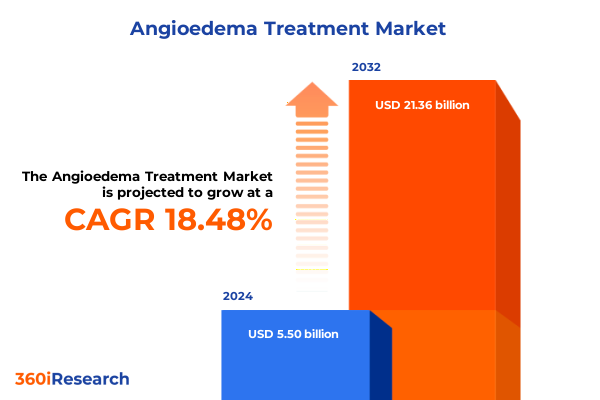
The Angioedema Treatment Market size was estimated at USD 6.47 billion in 2025 and expected to reach USD 7.62 billion in 2026, at a CAGR of 18.60% to reach USD 21.36 billion by 2032.
Understanding the Complex Global Challenges, Unmet Needs, and Critical Importance of Innovative Angioedema Treatment Across Hereditary, Acquired, and Idiopathic Conditions
Angioedema represents a heterogeneous group of conditions characterized by episodic, localized swelling of subcutaneous or submucosal tissues. While allergic triggers are common in acute presentations, hereditary, acquired, and idiopathic forms each pose distinct challenges for clinicians and patients alike. Hereditary angioedema (HAE) arises from genetic C1 inhibitor deficiencies or dysfunction, leading to unregulated bradykinin production. Acquired angioedema often develops secondary to lymphoproliferative disorders or autoantibodies that impair C1 inhibitor activity, and idiopathic angioedema remains a diagnosis of exclusion when no clear etiology can be identified. Together, these conditions can precipitate life-threatening airway compromise, severe abdominal pain, and significant quality-of-life impairment, underscoring an urgent need for effective management strategies.
Although constituting a rare disease category, HAE affects approximately 1.84 to 2.67 individuals per 100,000 in the United States, with global prevalence estimates ranging between 1 and 2 per 100,000 population. These figures exceed earlier assumptions, as claims-based studies have identified up to 2.67 cases per 100,000 when all HAE subtypes are considered and projected more than 9,500 diagnosed patients nationally before adjustment for Medicare representation. Emergency department visits for angioedema account for hundreds of thousands of encounters annually, driven by both hereditary and allergic variants of the disease, and demonstrate the broad burden of acute attacks on healthcare systems and families alike.
Recognizing the multifaceted nature of angioedema and the severe impact of unmitigated attacks, stakeholders ranging from academic researchers to industry innovators have accelerated efforts to develop targeted therapies. Improved understanding of the bradykinin pathway over the past decade has yielded new therapeutic classes, while heightened patient advocacy has led to earlier diagnosis and more personalized care pathways. As the landscape continues to evolve, a clear comprehension of the underlying disease mechanisms, patient segments, and care delivery environments is essential for guiding future innovation and ensuring optimal patient outcomes.
Identifying Transformational Changes in Angioedema Treatment Fueled by Breakthrough Therapies, Digital Tools, and Evolving Clinical Guidelines
The angioedema treatment paradigm has undergone a profound transformation in recent years, driven by breakthroughs in molecular targeting, evolving regulatory frameworks, and the integration of digital health solutions. First, the transition from broad-spectrum therapies toward highly specific agents has redefined prophylactic approaches. Targeted monoclonal antibodies against plasma kallikrein and bradykinin receptor antagonists have enabled clinicians to tailor long-term preventive regimens, reducing attack frequency and improving patient quality of life. Concurrently, the successful development of oral kallikrein inhibitors has challenged the longstanding notion that effective angioedema prophylaxis must involve intravenous or subcutaneous administration.
In parallel, on-demand management strategies have expanded beyond traditional injectable interventions. Oral on-demand therapies designed to act rapidly at the onset of symptoms are now poised to revolutionize acute care by offering greater convenience and enhanced patient autonomy. Moreover, the emergence of patient-centric digital applications for symptom tracking, adherence monitoring, and telemedicine consultations has strengthened the continuum of care, allowing for real-time data sharing between patients and providers and fostering earlier intervention.
Guideline development has kept pace with these advances, incorporating evidence-based recommendations that emphasize prompt on-demand treatment at the first sign of an attack, routine home self-administration, and the strategic use of prophylactic therapies for high-risk scenarios. Leading expert consensus now prioritizes therapies such as subcutaneous C1 inhibitors and monoclonal antibodies for long-term prophylaxis, with oral kallikrein inhibitors also recognized as first-line options. These collective shifts underscore a new era in angioedema management-one defined by precision targeting, patient empowerment, and seamless integration of technology into every aspect of care.
Analyzing the 2025 Impact of Expanded U.S. Tariffs on Medical Imports and Their Broad Implications for the Angioedema Treatment Supply Chain
Trade policy changes implemented by the Office of the United States Trade Representative under Section 301 have introduced significant tariff increases on critical medical imports, directly affecting the angioedema treatment supply chain. As of January 1, 2025, additional duties on rubber medical and surgical gloves rose to 50 percent, while certain respirators and face masks also transitioned from 7.5 to 25 percent before a further scheduled increase. Likewise, syringes and needles have borne a 100 percent tariff since September 27, 2024, creating upward cost pressures for injectable therapies and emergency care delivery. These measures reflect a broader strategic emphasis on reshoring domestic manufacturing capabilities and safeguarding national security interests by discouraging overreliance on single-country supply sources.
While pharmaceutical manufacturers and importers have petitioned for expanded exclusions, the USTR has extended certain exemptions only until August 31, 2025, leaving uncertainty for medical device and drug packaging components beyond that date. Industry associations have voiced concerns that sustained high tariffs could impede access to essential delivery systems and increase out-of-pocket costs for patients, potentially undermining recent gains in treatment adherence and home care utilization. Conversely, proponents argue that these policies may galvanize investment in U.S.-based production facilities, foster advanced manufacturing technologies, and ultimately enhance supply chain resilience.
Adding complexity to the environment, political discourse around potential future tariffs on pharmaceutical ingredients and finished products suggests that additional measures could emerge pending new national security assessments. Stakeholders must therefore weigh near-term cost impacts against longer-term supply chain diversification objectives as they navigate this evolving tariff landscape and its downstream implications for angioedema therapy availability and affordability.
Leveraging Precision Insights Across Drug Class, Administration Route, Disease Type, End User, and Distribution Channels in Angioedema Treatment Market Segmentation
An in-depth segmentation analysis reveals how heterogeneous patient populations, diversified treatment modalities, and varied distribution networks coalesce to shape the angioedema therapeutic arena. Within the classification of treatments by mechanism of action, bradykinin receptor antagonists, C1 esterase inhibitors, and kallikrein inhibitors each occupy distinct roles in managing acute attacks, short-term prophylaxis, and long-term prevention, respectively. These drug class differences inform physician prescribing patterns, patient preferences, and payer reimbursement criteria, while also influencing the prioritization of research and development pipelines.
Administration route segmentation further delineates the market into injectable and oral options, reflecting divergent patient experiences and clinical considerations. Injectable therapies continue to dominate acute intervention and some prophylactic regimens due to their rapid onset of action and well-established safety profiles. However, oral formulations are gaining traction for their convenience, improved adherence potential, and reduced burden on self-administration capabilities, making them particularly attractive for pediatric and remote-care settings.
Disease type stratification-acquired, hereditary, and idiopathic-underscores the nuanced clinical presentations and diagnostic pathways that guide treatment decisions. Hereditary forms often demand specialized long-term prophylactic strategies, whereas acquired cases may respond more readily to on-demand therapies once underlying triggers are addressed. Idiopathic angioedema, characterized by recurrent attacks without identifiable etiology, poses diagnostic and management challenges but also represents an area where personalized treatment algorithms can yield significant quality-of-life improvements.
From the perspective of end users, home care settings have emerged as a pivotal venue for both prophylactic and on-demand administration, buoyed by patient training programs and expanded self-administration approvals. Nonetheless, hospitals and specialty clinics remain critical for initial diagnosis, severe attack management, and complex cases requiring multidisciplinary interventions. Finally, the distribution channel landscape-comprised of hospital pharmacies, online pharmacies, and retail outlets-reflects shifting procurement models that prioritize rapid access, home delivery, and streamlined reimbursement processes to support timely therapy initiation and continuity of care.
This comprehensive research report categorizes the Angioedema Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Drug Class
- Administration Route
- Disease Type
- End User
- Distribution Channel
Exploring Distinct Regional Dynamics and Growth Drivers in the Americas, Europe Middle East & Africa, and Asia-Pacific Angioedema Treatment Markets
Regional market dynamics for angioedema treatment exhibit considerable diversity, driven by variations in regulatory environments, healthcare infrastructure, and patient awareness. In the Americas, the United States leads with a robust ecosystem characterized by fast-track regulatory pathways, widespread specialist networks, and a strong tradition of patient advocacy organizations. These factors have fostered rapid uptake of innovative therapies and comprehensive home care programs, while payer-driven demand for cost-effectiveness has spurred value-based contracting and outcome-based reimbursement models.
In Europe, Middle East & Africa, growth is propelled by harmonized regulatory frameworks under the European Medicines Agency, national healthcare system negotiations, and the gradual expansion of awareness campaigns in emerging Middle Eastern and African markets. Reimbursement policies vary markedly across countries, creating pockets of accelerated access alongside areas where budget constraints and pricing negotiations delay product launches. Nonetheless, patient registries and cross-border collaborations have begun to standardize care pathways, enabling broader adoption of prophylactic and on-demand treatment regimens.
The Asia-Pacific region offers a compelling frontier for future growth, as heightened disease recognition, expanding specialty care centers, and government-driven initiatives to strengthen rare disease policies converge. Countries such as Japan and Australia have established orphan drug incentives and patient support networks, while emerging markets in China and India are ramping up domestication of manufacturing and investments in local clinical trial capabilities. These developments, coupled with a growing middle-class population and improving insurance coverage, signal significant opportunities for companies seeking to broaden their footprint and address unmet needs across diverse healthcare settings.
This comprehensive research report examines key regions that drive the evolution of the Angioedema Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Leading Industry Players’ Strategies, Product Innovations, and Competitive Positioning in the Angioedema Treatment Landscape
The competitive landscape for angioedema treatment features a mix of established pharmaceutical giants and emerging biotechnology innovators, each aiming to differentiate through novel mechanisms, formulation enhancements, and strategic collaborations. BioCryst Pharmaceuticals has positioned itself at the forefront with ORLADEYO® (berotralstat), an oral plasma kallikrein inhibitor that gained full FDA approval for adult and pediatric prophylaxis, and recently secured Priority Review designation for expanded use in younger pediatric cohorts, signaling strong clinical momentum in the prophylactic segment. Meanwhile, KalVista Pharmaceuticals achieved a major milestone with FDA approval of its sebetralstat formulation, rebranded as Ekterly, marking the first oral on-demand treatment option for acute hereditary angioedema attacks and unlocking new patient-centric pathways for immediate symptom relief.
On the recombinant protein front, Pharming Group’s RUCONEST® has maintained a leading position as the only plasma-free C1 esterase inhibitor for acute attacks, benefiting from 12 years of FDA-granted exclusivity and reinforcing its role in settings where recombinant therapies are preferred. Shire, now part of Takeda, pioneered icatibant (Firazyr®), a bradykinin B2 receptor antagonist that enabled self-administration in emergency scenarios and laid the groundwork for subsequent bradykinin-targeting innovations. In parallel, CSL Behring continues to strengthen its portfolio with subcutaneous C1-INH products and invest in data generation for optimized prophylaxis and patient support services.
Each of these companies is leveraging differentiated strategies-ranging from value-based partnerships and digital engagement to global licensing agreements-to deepen market penetration and drive lifecycle expansions. As competition intensifies, emerging entrants and niche players are also exploring gene-silencing approaches, novel oral peptides, and combination regimens, underscoring the vibrancy and potential for continued innovation in the angioedema treatment field.
This comprehensive research report delivers an in-depth overview of the principal market players in the Angioedema Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Adverum Biotechnologies, Inc.
- Argenx NV
- AstraZeneca plc
- Attune Pharmaceuticals, Inc.
- Aurobindo Pharma Ltd.
- Bayer AG
- BioCryst Pharmaceuticals, Inc.
- Cipla Limited
- CSL Behring
- Dr. Reddy’s Laboratories Ltd.
- GlaxoSmithKline plc
- HAE Pharma LLC
- Intellia Therapeutics, Inc.
- Ionis Pharmaceuticals, Inc.
- KalVista Pharmaceuticals Ltd.
- Merck & Co., Inc.
- Novartis AG
- Otsuka Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Pharming Group N.V.
- Pharvaris Netherlands B.V.
- Sanofi S.A.
- Sobi
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
Actionable Strategies for Industry Leaders to Drive Growth, Enhance Access, and Accelerate Innovation in the Angioedema Treatment Ecosystem
To capitalize on evolving market dynamics and maximize patient impact, industry leaders should prioritize a multi-pronged approach that balances innovation with operational agility. First, aligning R&D investments with unmet clinical needs-particularly in pediatric populations, on-demand oral therapies, and rare disease subtypes-can unlock differentiated value propositions and secure accelerated regulatory pathways. Second, fostering strategic alliances with patient advocacy groups, payers, and digital health platforms will amplify outreach efforts, enhance adherence, and facilitate real-world data collection essential for value demonstration.
Operationally, companies should optimize supply chain resilience by diversifying sourcing strategies and engaging with domestic manufacturing initiatives, thereby mitigating exposure to potential tariff fluctuations and ensuring continuous product availability. Concurrently, embracing value-based contracting models that tie reimbursement to clinical outcomes can alleviate payer concerns and promote broader market access.
Finally, investing in robust market education campaigns targeting general practitioners, emergency department clinicians, and patient communities can heighten disease awareness, shorten diagnostic delays, and support timely intervention. By integrating these strategic imperatives, stakeholders can strengthen competitive positioning, drive sustainable growth, and ultimately enhance patient experiences across the angioedema treatment continuum.
Outlining Robust Research Methodology Combining Secondary Data, Expert Interviews, and Rigorous Quality Assurance to Inform Angioedema Treatment Research
This research harnessed a comprehensive methodology combining secondary data analysis, expert interviews, and rigorous quality assurance protocols. Secondary sources included peer-reviewed journals, regulatory filings, clinical trial registries, and official tariff documentation, ensuring a solid evidence foundation. Key opinion leaders and cross-functional stakeholders from pharmaceutical companies, healthcare providers, and patient advocacy organizations contributed qualitative insights, validating market trends and contextualizing quantitative findings.
To corroborate supply chain implications, publicly available policy briefs and trade commission notices were systematically reviewed, and thematic analysis was applied to extract relevant tariff changes. Segmentation analysis was conducted by mapping therapeutic classes, administration routes, disease types, end users, and distribution channels against global treatment patterns, enabling a granular view of market drivers and barriers.
Finally, data integrity was ensured through triangulation of diverse information streams and iterative feedback loops with subject matter experts. Findings were synthesized to deliver actionable insights, underpinned by transparent documentation of data sources, assumptions, and analytical frameworks, thereby reinforcing the report’s credibility and strategic relevance.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Angioedema Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Angioedema Treatment Market, by Drug Class
- Angioedema Treatment Market, by Administration Route
- Angioedema Treatment Market, by Disease Type
- Angioedema Treatment Market, by End User
- Angioedema Treatment Market, by Distribution Channel
- Angioedema Treatment Market, by Region
- Angioedema Treatment Market, by Group
- Angioedema Treatment Market, by Country
- United States Angioedema Treatment Market
- China Angioedema Treatment Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 954 ]
Summarizing Key Takeaways and Strategic Imperatives to Navigate the Evolving Angioedema Treatment Landscape with Confidence and Insight
The angioedema treatment landscape is characterized by robust innovation, shifting patient expectations, and evolving policy frameworks. Breakthrough therapies targeting kallikrein and bradykinin, alongside novel oral formulations, have redefined prophylactic and acute management paradigms. At the same time, U.S. trade measures in 2025 underscore the need for strategic supply chain diversification, while digital health solutions and guideline updates continue to elevate standards of care.
Market segmentation insights highlight the importance of tailored approaches across drug classes, administration routes, and patient subtypes, reinforcing the value of precision medicine in angioedema. Regional dynamics further elaborate the nuances of reimbursement policies and healthcare infrastructure, informing differentiated market entry and expansion strategies. Key companies are leveraging these insights to drive lifecycle management, strategic alliances, and real-world evidence generation.
Going forward, stakeholders who synergize clinical innovation with patient-centric engagement, operational resilience, and outcome-oriented reimbursement will be best positioned to navigate the complexities and unlock value. This synthesis of trends, challenges, and opportunities provides a clear roadmap for advancing angioedema care and delivering sustainable growth in a rapidly transforming market.
Initiating Your Next Steps Now to Engage With Ketan Rohom for Exclusive Access to the Comprehensive Angioedema Treatment Market Research Report
Embark on the definitive path to gaining an unparalleled understanding of the angioedema treatment market by reaching out directly to Ketan Rohom, Associate Director of Sales & Marketing, who stands ready to guide you through the wealth of insights contained in the comprehensive market research report.
By connecting with Ketan, you will unlock tailored access to in-depth analysis on emerging therapies, detailed segmentation data, and region-specific forecasts that can empower your strategic decision-making and accelerate your organization’s growth trajectory in the rapidly evolving angioedema landscape. Secure your competitive advantage and elevate your market intelligence by initiating a conversation today.

- How big is the Angioedema Treatment Market?
- What is the Angioedema Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




