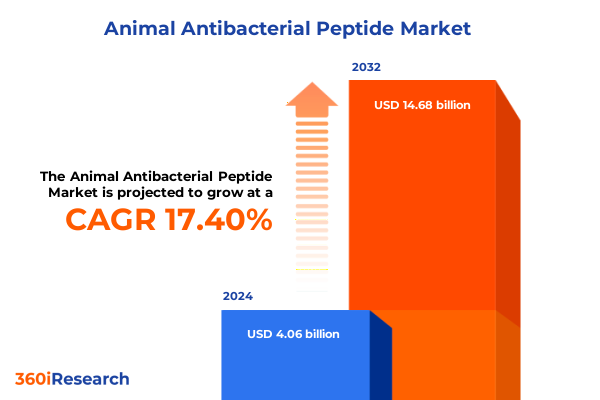
The Animal Antibacterial Peptide Market size was estimated at USD 4.69 billion in 2025 and expected to reach USD 5.42 billion in 2026, at a CAGR of 17.68% to reach USD 14.68 billion by 2032.
Pioneering a New Era in Animal Health with Antibacterial Peptides That Challenge Conventional Antibiotics and Drive Sustainable Practices
The quest for innovative disease management solutions in animal health has never been more critical. As global concerns around antimicrobial resistance intensify and regulatory bodies impose stricter controls on traditional antibiotics, the focus has turned decisively toward antibacterial peptides. These naturally occurring biomolecules and engineered analogues offer targeted mechanisms of action that disrupt bacterial membranes and impede pathogenic proliferation without fostering resistance the way conventional antibiotics do.
Initial research into antibacterial peptides revealed broad-spectrum activity against a range of Gram-positive and Gram-negative pathogens, alongside promising immunomodulatory effects that enhance host defenses. Over the last decade, advances in peptide engineering and large-scale biomanufacturing have matured these compounds from academic curiosities into commercially viable solutions. New formulation technologies now support oral, injectable, and feed-incorporated delivery, unlocking applications across aquaculture, livestock, and companion animal care.
Against this backdrop, stakeholders are reevaluating long-standing antibiotic use models. Transitioning toward antibacterial peptides not only aligns with emerging regulatory mandates but also addresses growing consumer demand for sustainably sourced protein. As the landscape rapidly transitions, early adopters of peptide-based interventions will set the standard for improved animal welfare, optimized production yields, and reduced environmental impact.
How Advances in Biotechnology, Regulatory Reforms, and Industry Collaborations Are Redefining the Animal Antibacterial Peptide Landscape Globally
Industry dynamics are shifting dramatically under the influence of three interrelated forces: biotechnological innovation, regulatory realignment, and strategic cross-sector partnerships. Breakthroughs in peptide synthesis have enabled precise control over molecular weight, hydrophobicity, and charge distribution, translating into enhanced antimicrobial efficacy and stability. These technical strides are complemented by novel formulation platforms, such as microencapsulation and sustained-release matrices, which extend bioactivity in vivo and permit seamless integration into existing animal husbandry protocols.
Moreover, regulators in major markets have unveiled revised guidelines that accelerate peptide approvals. By adopting adaptive licensing pathways and harmonizing safety assessments, authorities facilitate more rapid transitions from proof-of-concept studies to commercial launch. Simultaneously, the rise of contract development and manufacturing organizations specializing in peptide production has reduced time to market, offering end users expanded supply options and mitigating previous scale-up bottlenecks.
In parallel, collaborative ventures between feed producers, veterinary pharmaceutical companies, and research institutes have formed to co-develop application-specific peptide blends. These alliances leverage shared expertise in genomic screening, bioinformatics, and delivery systems to tailor solutions for growth promotion, prophylaxis, and targeted therapeutics. Consequently, what was once a niche innovation has transformed into a robust growth engine reshaping the future of animal health.
Assessing the Comprehensive Impact of 2025 U.S. Tariff Measures on Animal Antibacterial Peptide Supply Chains Costs and Domestic Production Strategies
Throughout 2025, U.S. trade policy has evolved in ways that directly influence the cost and availability of antibacterial peptides. Early in the year, the administration expanded ad valorem duties on chemical and pharmaceutical imports under a broad executive order targeting specific supply chain vulnerabilities. Subsequently, a Section 232 investigation into pharmaceutical ingredients introduced additional scrutiny on critical inputs, including peptides and precursor molecules. Further adjustments to tariff schedules imposed targeted duties on products sourced from select foreign markets, compelling end users to reassess global sourcing strategies.
As a result, companies importing synthetic peptides or key raw materials encountered escalated landed costs. This fiscal pressure has accelerated domestic investment in biomanufacturing capacity, while also encouraging vertical integration among feed producers and veterinary pharma firms. In parallel, rising import duties on long-chain synthetic peptides have prompted formulators to pivot toward medium-chain variants or to increase reliance on indigenous peptide libraries. These supply realignments, though disruptive in the short term, have sparked renewed interest in building resilient local supply networks.
Notably, the cumulative impact of these tariff measures is not uniform across segments. Naturally derived peptides, often produced domestically, enjoy relative insulation from import levies. Yet their higher extraction and purification costs continue to challenge commercial scalability. In contrast, synthetic peptides face ongoing headwinds from trade policy, prompting strategic sourcing shifts and input innovation to optimize total cost of ownership.
Unveiling Critical Market Segmentation Insights Revealing How Product Types, Applications, and End Users Shape the Antibacterial Peptide Sector Dynamics
A nuanced understanding of the antibacterial peptide market emerges through its foundational segmentation. In terms of product type, the field divides between naturally derived peptides sourced from animal or microbial origins and fully synthetic analogues. Naturally derived variants typically leverage innate antimicrobial properties, whereas synthetic peptides offer the design flexibility to adjust chain length for optimized stability and spectrum of activity. Among synthetic options, chain lengths span short, medium, and long formats, each tailored to address specific microbial targets and pharmacokinetic profiles.
From an application standpoint, formulations aim to support three primary objectives. Growth promotion seeks to enhance feed conversion ratios and weight gain, particularly in aquaculture and poultry, through subtherapeutic dosing strategies. Prophylactic use targets disease prevention and functions as vaccination adjuvant, bolstering immune response ahead of pathogen exposure. In established disease scenarios, therapeutic interventions employ peptides for infection treatment and for management of skin-related conditions, reducing reliance on conventional antibiotic regimens.
Finally, end-user segmentation reflects diverse operational environments. Aquaculture farms and feed manufacturers, including aquafeed and livestock feed producers, form the backbone of large-scale incorporation. Research institutes, covering both academic centers and biotech companies, spearhead discovery and optimization programs. Veterinary clinics, segmented into large animal and small animal practices, represent the primary channels for therapeutic applications, underscoring the versatility of peptide platforms across species and use cases.
This comprehensive research report categorizes the Animal Antibacterial Peptide market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- End User
Examining Regional Variations in Demand, Innovation and Regulatory Environments Driving Growth of Animal Antibacterial Peptides Across Key Global Markets
Regional markets exhibit distinct attributes driven by regulatory stringency, production capacity, and consumption patterns. In the Americas, a coastline-to-coast emphasis on antibiotic stewardship has accelerated peptide adoption in both livestock and aquaculture. Incentive programs and public–private partnerships have supported pilot programs integrating peptides into feed, while established contract manufacturing facilities ensure a resilient supply base within North America.
Across Europe, the Middle East, and Africa, stringent bans on antibiotic growth promoters in the European Union have catalyzed R&D initiatives in peptide alternatives. Collaborative research consortia spanning regulatory bodies and academic institutions advance harmonized safety standards, facilitating market entry. Meanwhile, emerging markets in the Gulf region and parts of Africa demonstrate growing demand for prophylactic peptides as livestock operations modernize and seek to mitigate disease outbreaks under challenging climatic conditions.
In Asia-Pacific, a combination of large-scale animal protein producers and rapid aquaculture expansion has driven significant peptide uptake. Local producers in China and India focus on cost-competitive manufacturing, whereas regulatory reforms in Australia and Japan emphasize quality assurance and residue monitoring. Collectively, these markets form a dynamic landscape where regional leadership arises from tailored policy frameworks and strategic domestic partnerships.
This comprehensive research report examines key regions that drive the evolution of the Animal Antibacterial Peptide market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Players and Strategic Partnerships Fueling Innovation and Competitive Differentiation in the Animal Antibacterial Peptide Market
A competitive cohort of multinational chemical companies, specialized biotech innovators, and contract development partners anchors the animal antibacterial peptide ecosystem. Established chemical conglomerates have leveraged existing fermentation and purification infrastructure to expand pipelines with naturally derived peptide products. At the same time, precision biotech firms harness advanced genomic screening and computational modeling to design synthetic analogues with enhanced potency and reduced immunogenicity.
Key strategic partnerships between feed manufacturers and peptide developers have facilitated co-creation of custom formulations, accelerating route-to-market for application-specific blends. Contract development and manufacturing organizations have scaled pilot processes to commercial volumes, offering turnkey solutions from gene synthesis through peptide purification and formulation. These alliances not only distribute risk but also enable smaller innovators to tap into established quality and regulatory frameworks.
Moreover, a wave of venture capital and corporate investment has targeted next-generation peptide platforms, particularly those blending antimicrobial functionality with immunomodulatory benefits. This influx of funding spurs ongoing pipeline expansion, with late-stage candidates moving into field trials and regulatory submissions. As these candidates advance, the competitive landscape will further intensify, rewarding companies that combine robust R&D pipelines with strategic channel partnerships.
This comprehensive research report delivers an in-depth overview of the principal market players in the Animal Antibacterial Peptide market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AB Vista Limited
- AgroBioTek Laboratories LLC
- Animal Medics
- Anitox Corporation
- Biogenesis Bagó S.A.
- Biosynth Ltd
- Boehringer Ingelheim International GmbH
- Cargill, Incorporated
- Dechra Pharmaceuticals PLC
- Eco Animal Health Ltd.
- Elanco Animal Health Incorporated
- Esco Aster Pte. Ltd.
- Genera Inc.
- Huvepharma S.A.
- ImmuCell Corporation
- Kemin Industries, Inc.
- Merck & Co., Inc.
- Neogen Corporation
- Novozymes A/S
- Phibro Animal Health Corporation
- Vetbiochem India Pvt Ltd.
- Vetoquinol S.A.
- Zoetis Inc.
- Zomedica Pharmaceuticals Corp.
Actionable Recommendations for Industry Leaders to Navigate Tariff Challenges, Optimize Supply Chains, and Accelerate Adoption of Animal Antibacterial Peptides
Industry leaders should act decisively to mitigate trade and supply chain risks by diversifying sourcing across domestic and selected low-tariff regions. Establishing strategic alliances with contract manufacturers capable of producing both synthetic and naturally derived peptides will enhance supply resilience and reduce exposure to import levies. Concurrently, companies must invest in formulation specialists to develop feed-friendly delivery formats and injection-compatible carriers that meet end-user handling requirements.
To strengthen market positioning, organizations should pursue joint validation studies with research institutes and key end users. Co-developed demonstration trials that showcase growth performance, prophylactic efficacy, and therapeutic outcomes will drive broader acceptance and facilitate regulatory filings. Furthermore, integrating real-world data from livestock operations into value propositions can underscore cost efficiencies and animal welfare benefits relative to traditional antibiotics.
Finally, to capitalize on the increasing demand, leaders should adopt tiered pricing strategies aligned with regional regulatory incentives and purchasing capacities. Tailoring commercial models to support small-scale producers in emerging markets while delivering premium, high-potency formulations to advanced veterinary practices will optimize market coverage and revenue streams.
Detailing the Robust Research Methodology Employed to Ensure Comprehensive, Accurate and Actionable Insights into the Animal Antibacterial Peptide Market
The research underpinning this report employed a comprehensive multi-method approach to ensure depth, accuracy, and strategic relevance. Primary data collection involved interviews with C-level executives, R&D heads, and procurement specialists across feed manufacturers, veterinary clinics, and biotechnology firms. These firsthand perspectives validated emerging trends in product formulation, application preferences, and supply chain configurations.
Secondary research encompassed a thorough review of regulatory filings, government trade notices, and peer-reviewed studies focused on peptide efficacy and safety. Analysis of tariff schedules and government proclamations provided clarity on the evolving trade environment impacting peptide imports and domestic manufacturing incentives. Market participants’ public statements, investor presentations, and patent filings were also evaluated to gauge innovation trajectories and competitive positioning.
Finally, quantitative modeling of supply and demand factors integrated historical adoption rates with scenario analyses reflecting variable tariff regimes. This blend of qualitative and quantitative techniques ensured that the insights offer a holistic view of the market landscape while preserving actionable specificity for strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Animal Antibacterial Peptide market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Animal Antibacterial Peptide Market, by Product Type
- Animal Antibacterial Peptide Market, by Application
- Animal Antibacterial Peptide Market, by End User
- Animal Antibacterial Peptide Market, by Region
- Animal Antibacterial Peptide Market, by Group
- Animal Antibacterial Peptide Market, by Country
- United States Animal Antibacterial Peptide Market
- China Animal Antibacterial Peptide Market
- Competitive Landscape
- List of Figures [Total: 15]
- List of Tables [Total: 1590 ]
Synthesizing Key Findings to Highlight the Strategic Importance and Future Trajectory of Animal Antibacterial Peptide Innovations in Animal Health
Our analysis illuminates how antibacterial peptides are transitioning from promising research initiatives to vital tools in modern animal health management. The convergence of innovative peptide design, adaptive regulatory pathways, and strategic partnerships has created an ecosystem primed for expansion. Meanwhile, U.S. trade policies in 2025 have stimulated domestic production investments and reshaped global supply chain strategies, underscoring the imperative for agility in sourcing and formulation.
Segment-level insights reveal that both naturally derived and synthetic peptides have distinct roles across growth promotion, disease prevention, and targeted therapy. End-user demand is heterogeneous, with feed manufacturers and aquaculture farms driving large-scale incorporation, and veterinary practices championing therapeutic and prophylactic use in diverse animal species. Regionally, each geography presents unique regulatory catalysts and market entry considerations that will define localized adoption curves.
Looking forward, companies that align R&D pipelines with end-user needs, fortify supply chain resilience, and navigate tariff challenges will lead market expansion. The collective momentum around sustainable animal protein production and antibiotic stewardship positions antibacterial peptides as indispensable components of 21st-century agriculture and veterinary care.
Secure Your Competitive Advantage in Animal Health by Connecting with Ketan Rohom to Access the Comprehensive Animal Antibacterial Peptide Market Report Today
To secure your strategic foresight and maintain a competitive edge in the rapidly evolving animal antibacterial peptide space, take the next step by engaging directly with Ketan Rohom, the Associate Director of Sales & Marketing. His in-depth expertise and nuanced understanding of livestock, aquaculture, and veterinary applications will help you tailor a solution that aligns with your unique organizational objectives.
By initiating a detailed discussion with Ketan Rohom, you gain privileged access to the full suite of insights, comprehensive data analyses, and proprietary intelligence featured in the market research report. Whether you seek to streamline supply chains, navigate complex regulatory frameworks, or identify high-potential partnership opportunities, his guidance will ensure you derive maximum value from the report’s findings.
Don’t let shifting market dynamics and emerging tariff challenges undermine your ability to capitalize on the transformative potential of antibacterial peptides. Connect with Ketan to arrange a personalized consultation, explore flexible licensing options, and secure immediate access to the in-depth research that will inform your strategic roadmap in 2025 and beyond.

- How big is the Animal Antibacterial Peptide Market?
- What is the Animal Antibacterial Peptide Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




