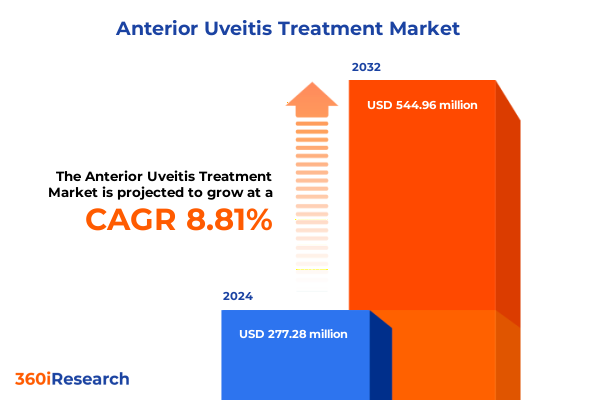
The Anterior Uveitis Treatment Market size was estimated at USD 302.01 million in 2025 and expected to reach USD 330.82 million in 2026, at a CAGR of 8.79% to reach USD 544.95 million by 2032.
Exploring the Fundamental Pathophysiology and Clinical Treatment Paradigms of Anterior Uveitis to Contextualize Market Dynamics
Anterior uveitis presents a complex interplay of immunological, infectious, and idiopathic factors that challenge clinicians and industry alike. Characterized by inflammation of the iris and ciliary body, this condition can manifest acutely with pain, photophobia, and vision impairment or chronically with subtle symptoms leading to delayed diagnosis. The multifactorial etiology encompasses autoimmune disorders, infectious agents, and trauma, underscoring the importance of a comprehensive diagnostic and therapeutic framework that addresses both inflammation control and prevention of long-term complications.
Within this context, treatment paradigms have evolved from broad immunosuppression to targeted interventions that optimize efficacy while minimizing systemic exposure. Non-pharmacological approaches such as laser therapy and surgical intervention have become viable adjuncts, particularly in refractory cases, while pharmacological strategies emphasize the strategic use of corticosteroids, biologics, immunosuppressants, and NSAIDs. This introduction sets the stage for a deeper examination of the forces reshaping the anterior uveitis market landscape and highlights the critical need for strategic alignment among developers, payers, and providers.
Identifying the Emerging Therapeutic Innovations and Shifting Competitive Landscape Transforming Anterior Uveitis Management Practices
The landscape of anterior uveitis management is undergoing a transformative shift driven by breakthroughs in biologic therapies and advances in local drug delivery systems. Where corticosteroids once dominated the treatment algorithm, the advent of monoclonal antibodies and receptor antagonists targeting specific inflammatory mediators has introduced new paradigms for long-term disease control. Concurrently, innovations in sustained-release implantable devices are redefining patient adherence and clinical outcomes by enabling localized administration through intravitreal or periocular routes, thereby reducing systemic exposure and associated adverse events.
Moreover, the expanding use of minimally invasive laser and surgical procedures reflects a growing recognition of non-pharmacological modalities as integral components of comprehensive care. Regulators have responded with accelerated approval pathways for therapies demonstrating clear benefits in preventing vision-threatening complications. In turn, manufacturers are forming strategic alliances and deploying real-world evidence programs to substantiate long-term safety and effectiveness. As a result, stakeholders across the value chain are recalibrating investment priorities, with a pronounced focus on personalized medicine, digital health integration, and scalable delivery platforms.
Assessing the Far Reaching Consequences of 2025 United States Tariff Measures on Anterior Uveitis Treatment Supply Chains and Costs
In 2025, the United States implemented targeted tariff measures on imported pharmaceutical ingredients and specialized ophthalmic devices, exerting notable pressure on anterior uveitis treatment supply chains. These tariffs have increased input costs for high-value biologics and implantable delivery technologies, prompting manufacturers to reevaluate sourcing strategies and explore localized production or tariff-exempt substitutes. Consequently, cost containment efforts have intensified, and price negotiations with payers have become more complex, particularly for novel therapies that depend on proprietary active ingredients.
Beyond direct cost impacts, the tariffs have incentivized greater vertical integration among contract development and manufacturing organizations, aiming to secure supply continuity and mitigate regulatory exposure. Payers and providers are increasingly scrutinizing total cost of care, emphasizing value-based contracting and outcome-oriented reimbursement models to offset higher unit prices. As a result, the anterior uveitis market is witnessing a recalibration of the competitive landscape, with an emphasis on differentiated clinical benefits, cost-effective local manufacturing, and evidence generation to support premium pricing under constrained procurement environments.
Unveiling Detailed Segmentation Insights to Illuminate Patient Centric Market Opportunities Across Treatment Types Drug Classes and Distribution Channels
A nuanced segmentation framework reveals divergent drivers of growth and adoption across treatment modalities. Based on treatment type, non-pharmacological interventions such as laser therapy and surgical procedures attract interest for patients with refractory or recurrent inflammation, while pharmacological approaches leverage corticosteroids for rapid anti-inflammatory effect, immunosuppressants for broader immune modulation, biologics for targeted cytokine inhibition, and NSAIDs for adjunctive symptom relief. Simultaneously, examining drug classes underscores the differentiated risk-benefit profiles of each category, with biologics commanding higher development costs and regulatory scrutiny, corticosteroids remaining a staple for acute management, and immunosuppressants catering to chronic, systemic cases.
Focusing on route of administration, intravitreal delivery offers controlled local exposure, periocular injections balance efficacy and invasiveness, systemic dosing addresses multisite inflammation, and topical formulations provide convenient self-administration albeit with variable ocular penetration. Distribution channels further influence market dynamics, as hospital pharmacies stock high-complexity therapies, retail pharmacies serve community-based prescriptions, and online pharmacies enable direct-to-patient models. Finally, end-user segmentation highlights the different points of care, where ambulatory surgery centers drive procedural volume, clinics emphasize outpatient management, and hospitals handle severe or complicated presentations. Together, these overlapping segments delineate clear pathways for targeted product positioning and tailored commercial strategies.
This comprehensive research report categorizes the Anterior Uveitis Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Drug Class
- Route Of Administration
- Distribution Channel
- End User
Examining Regional Market Dynamics Across the Americas Europe Middle East Africa and Asia Pacific to Highlight Growth Variances in Anterior Uveitis Care
Geographically, the Americas region demonstrates robust adoption of advanced biologics and localized delivery systems, underpinned by favorable reimbursement frameworks and expansive clinical trial activity in the United States and Canada. Shifting payer models have accelerated the transition toward outcome-based contracting, particularly for high-cost interventions. Meanwhile, Europe, the Middle East, and Africa present a heterogeneous landscape: Western Europe offers mature markets with established clinical guidelines and price controls, whereas emerging markets in the Middle East and North Africa are characterized by growing public health investment and rising prevalence of autoimmune conditions driving demand for specialized therapies.
In contrast, the Asia-Pacific region exhibits dynamic growth fueled by increased healthcare infrastructure spending and expanding ophthalmology networks across China, India, Southeast Asia, and Australia. Local regulatory agencies have begun to align more closely with international standards, enabling cross-border clinical collaborations and faster market entry. Nonetheless, pricing pressures and generic competition remain significant challenges in cost-sensitive markets. As a result, stakeholders are prioritizing tiered product portfolios and adaptive pricing strategies to balance innovation with affordability across diverse patient populations.
This comprehensive research report examines key regions that drive the evolution of the Anterior Uveitis Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Key Industry Players Driving Innovation and Strategic Collaborations in the Anterior Uveitis Therapeutics Market Landscape
Several leading biopharmaceutical and ophthalmic device manufacturers have established commanding presences in the anterior uveitis arena. AbbVie continues to leverage its immunology expertise, focusing on pipeline monoclonal antibodies that target interleukin pathways implicated in ocular inflammation. Roche has expanded its localized delivery portfolio with sustained-release implants designed for extended intraocular residence and precise drug release kinetics. Novartis has deepened its footprint through strategic acquisitions of biotech innovators specializing in small-molecule immunosuppressants and novel anti-inflammatory compounds.
Device specialists such as Alcon and Bausch + Lomb are pioneering next-generation laser platforms and minimally invasive surgical instruments to augment pharmacological regimens. Meanwhile, Regeneron Biopharmaceuticals has entered the uveitis vertical by repurposing anti-angiogenic treatments for inflammatory applications. Smaller biotech firms are also making strides, particularly those advancing cell-based therapies and gene modulation approaches. Across the industry, partnerships between established players and emerging biotech ventures underscore a collaborative ethos aimed at accelerating translational research and optimizing patient outcomes.
This comprehensive research report delivers an in-depth overview of the principal market players in the Anterior Uveitis Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Aciont Inc.
- Ajanta Pharma Ltd
- Alcon Inc.
- Aldeyra Therapeutics, Inc.
- Alimera Sciences Inc.
- Amgen Inc.
- Bausch Health Companies Inc.
- Cipla Ltd.
- Clearside Biomedical, Inc.
- Enzo Biochem Inc.
- Eyegate Pharmaceuticals, Inc
- Fera Pharmaceuticals LLC
- HanAll Biopharma
- Imprimis Pharmaceuticals, Inc.
- Kiora Pharmaceuticals, Inc.
- L V PRASAD EYE INSTITUTE
- Lux Biosciences, Inc.
- Novartis International AG
- Oculis SA
- Pfizer Inc.
- Regeneron Pharmaceuticals, Inc.
- Santen Pharmaceutical Co., Ltd.
- Sirion Therapeutics, Inc.
- Sun Pharmaceutical Industries Ltd.
- Tarsier Pharma Ltd
- Xoma Corporation
Actionable Strategic Recommendations for Industry Leaders to Navigate Regulatory Complexities and Capitalize on Unmet Needs in Anterior Uveitis Care
Industry leaders should prioritize the development of targeted biologic agents that address unmet inflammatory pathways while maintaining a clear value proposition for payers through robust real-world evidence and health economic studies. Simultaneously, enhancing manufacturing resilience through regional production hubs will mitigate tariff impacts and ensure supply continuity. Leveraging integrated digital health platforms to monitor patient adherence and clinical outcomes can differentiate offerings and support value-based contracting arrangements.
Furthermore, partnering with ophthalmic surgery centers and specialty clinics to co-develop protocols for combination therapies will foster adoption of integrated treatment regimens. Engaging with regulatory agencies early in development to secure accelerated approval designations or orphan drug status can expedite time to market. Finally, tailoring market entry strategies by aligning pricing and service models with regional healthcare infrastructure and reimbursement landscapes will unlock opportunities in cost-sensitive markets without compromising on innovation.
Outlining Rigorous Research Methodologies and Data Collection Approaches Underpinning Comprehensive Analysis of Anterior Uveitis Treatment Trends
This analysis integrates primary research, secondary data synthesis, and expert insights to deliver a comprehensive view of the anterior uveitis treatment landscape. Primary research comprised in-depth interviews with ophthalmologists, key opinion leaders, payers, and supply chain executives to validate market drivers, barriers, and clinical adoption patterns. Secondary research drew from peer-reviewed publications, regulatory dossiers, and real-world databases to contextualize clinical outcomes and therapeutic trends.
Data triangulation was employed to reconcile disparate information sources, ensuring consistency and reliability. Geographic and segment-specific analyses utilized a combination of hospital procurement records, specialty pharmacy data, and proprietary market intelligence. Quality control measures included cross-validation with publicly available registries and conference abstracts. The result is a robust methodological framework that underpins the insights presented, enabling stakeholders to make informed strategic decisions with confidence.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Anterior Uveitis Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Anterior Uveitis Treatment Market, by Treatment Type
- Anterior Uveitis Treatment Market, by Drug Class
- Anterior Uveitis Treatment Market, by Route Of Administration
- Anterior Uveitis Treatment Market, by Distribution Channel
- Anterior Uveitis Treatment Market, by End User
- Anterior Uveitis Treatment Market, by Region
- Anterior Uveitis Treatment Market, by Group
- Anterior Uveitis Treatment Market, by Country
- United States Anterior Uveitis Treatment Market
- China Anterior Uveitis Treatment Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1272 ]
Synthesizing Insights to Provide a Clear Perspective on Future Trajectories and Investment Imperatives in the Anterior Uveitis Treatment Arena
Throughout this report, the convergence of biologic innovations, localized delivery advancements, and integrated care models signals a clear inflection point in anterior uveitis therapy. The strategic emphasis on precision medicine and value-based care will continue to shape development pipelines and reimbursement policies. Concurrently, evolving tariff regimes and regional infrastructure disparities will influence supply dynamics and adoption patterns, underscoring the importance of adaptive commercial strategies.
Looking ahead, the ocular inflammation segment presents fertile ground for collaboration across pharmaceutical, device, and digital health domains. Investment in biomarkers and patient stratification tools will enhance clinical trial design and market targeting. The maturation of real-world evidence networks offers an avenue to substantiate long-term safety and comparative effectiveness. Ultimately, the organizations that harness these insights and execute on data-driven, patient-centric approaches will secure leadership positions in the next generation of anterior uveitis treatments.
Compelling Invitation to Engage with Ketan Rohom for Access to the Definitive Anterior Uveitis Treatment Market Research Report
Engaging with Ketan Rohom, Associate Director of Sales & Marketing at 360iResearch, ensures your organization gains immediate strategic value from this in-depth anterior uveitis treatment analysis. His expertise and leadership in delivering comprehensive market insights will guide you in tailoring your development, distribution, and commercialization initiatives to the unique dynamics of the anterior uveitis space. By reaching out, you will access supplementary data sets, customized competitive benchmarking, and detailed briefing sessions designed to accelerate your decision-making and shorten your time to market.
Seize the opportunity to secure this definitive market research report and position your organization at the forefront of anterior uveitis treatment innovation. Connect with Ketan Rohom today to discuss licensing options, enterprise-wide deployment, and bespoke advisory services that will empower you to capitalize on emerging opportunities, mitigate regulatory and tariff-related risks, and build lasting competitive advantage in this rapidly evolving therapeutic segment.

- How big is the Anterior Uveitis Treatment Market?
- What is the Anterior Uveitis Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




