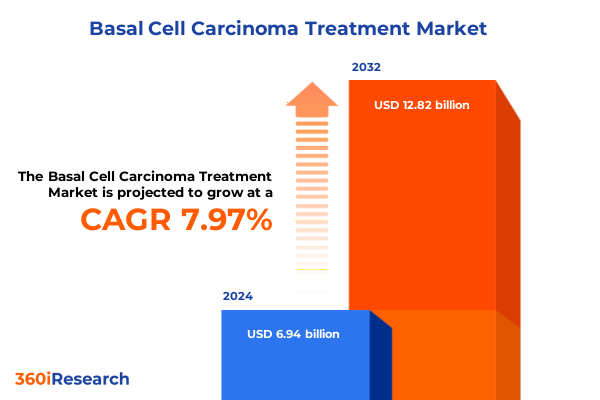
The Basal Cell Carcinoma Treatment Market size was estimated at USD 7.40 billion in 2025 and expected to reach USD 7.90 billion in 2026, at a CAGR of 8.15% to reach USD 12.82 billion by 2032.
Understanding the evolving challenges and clinical opportunities shaping the future of basal cell carcinoma treatment modalities for broad patient cohorts
Basal cell carcinoma (BCC) stands as the most prevalent form of skin cancer worldwide, with estimates indicating that nearly 80 percent of the more than 5.4 million nonmelanoma skin cancers diagnosed annually in the United States are basal cell carcinomas. While mortality rates remain comparatively low, BCC can inflict significant morbidity, disfigurement, and healthcare costs if not detected and treated early. The rising incidence of BCC over recent decades is attributed to factors such as increased ultraviolet exposure, aging populations, and enhanced diagnostic vigilance.
As the clinical and economic burden of BCC grows, stakeholders across the healthcare continuum are intensifying efforts to refine diagnostic accuracy, optimize therapeutic pathways, and improve patient outcomes. From traditional surgical excision and Mohs micrographic surgery to emerging pharmacologic interventions targeting key oncogenic pathways, the therapeutic armamentarium has expanded substantially. This executive summary synthesizes the latest evidence, market forces, and regulatory dynamics shaping BCC treatment, offering a foundational overview for decision makers seeking to navigate this complex environment.
Emerging paradigm shifts revolutionizing basal cell carcinoma care through innovation in diagnostics, therapies, and patient-centered treatment approaches
The BCC treatment landscape is undergoing a profound transformation driven by advances in immunotherapy, noninvasive diagnostics, and next-generation therapeutic modalities. In 2021, the Food and Drug Administration granted full approval to the PD-1 inhibitor cemiplimab-rwlc for patients with locally advanced basal cell carcinoma previously treated with hedgehog pathway inhibitors, marking the first immunotherapy option for this indication. This milestone has opened new avenues for patients intolerant of or refractory to existing targeted treatments.
Simultaneously, noninvasive imaging techniques such as in vivo reflectance confocal microscopy (RCM) are redefining diagnostic workflows by delivering cellular-level resolution without the need for excisional biopsy. Meta-analytic evidence demonstrates that RCM achieves pooled sensitivity and specificity exceeding 90 percent for primary BCC diagnosis, significantly reducing unnecessary biopsies and streamlining clinical decision making. Deep learning algorithms applied to RCM images are further enhancing diagnostic precision, heralding a future of AI-augmented dermatologic care.
Photodynamic therapy (PDT) continues to evolve as a noninvasive option, particularly for low-risk BCC subtypes. Randomized controlled trials comparing 5-aminolevulinic acid and methyl aminolevulinate formulations have confirmed PDT’s favorable cosmetic outcomes and acceptable efficacy, positioning it as an alternative to surgery for select lesions. Combined with emerging jet-injection and daylight protocols, PDT is poised to expand its clinical utility.
These paradigm shifts are complemented by digital health interventions, including teledermatology platforms that extend specialist reach into underserved regions and mobile applications that empower patients to monitor lesion changes remotely. As the industry moves toward personalized, patient-centric care, the integration of immunotherapies, precision diagnostics, and digital tools will be instrumental in improving outcomes and controlling costs.
Assessing the cumulative effects of 2025 U.S. tariff measures on supply chain resilience and cost structures in basal cell carcinoma therapy delivery
In 2025, the United States implemented a series of tariff measures that are reshaping global pharmaceutical supply chains and exerting upward pressure on the cost of BCC therapies. A high-profile analysis by Ernst & Young projected that a 25 percent U.S. tariff on pharmaceutical imports could raise annual drug costs by as much as $51 billion and increase consumer prices by nearly 13 percent if passed on by distributors. These duties particularly affect active pharmaceutical ingredients sourced from Europe, which accounted for nearly three-quarters of U.S. pharmaceutical imports in 2023.
Complementing this, tariffs of up to 25 percent on APIs from China and 20 percent on those from India have been imposed, targeting the foundational building blocks for oncology medications, including Hedgehog pathway inhibitors. Such levies have immediate inflationary effects on production costs, prompting drugmakers to reconsider global sourcing strategies and accelerate investments in domestic manufacturing. While this policy is intended to bolster onshore capacity, the transition entails significant capital expenditure and time before supply chains can be fully realigned.
The cumulative impact of these measures is reverberating through procurement budgets, payer negotiations, and formulary decisions, with downstream effects on patient access. Healthcare providers and manufacturers must therefore navigate an increasingly complex tariff landscape, balancing cost containment with the imperative to maintain a robust and reliable supply of critical BCC therapies.
Uncovering segmentation insights that illuminate how treatment types, drug classes, end users, and patient profiles shape basal cell carcinoma treatment strategies
Insights derived from detailed segmentation analyses highlight the multifaceted nature of the BCC treatment ecosystem. Based on treatment type, non-surgical therapies such as cryotherapy, immunotherapy, photodynamic and topical interventions coexist alongside surgical procedures like Mohs micrographic surgery and standard excisional techniques, each addressing distinct risk profiles and patient preferences. Within drug class segmentation, Hedgehog pathway inhibitors-principally vismodegib and sonidegib-represent a targeted pharmacologic category for advanced and inoperable lesions. End users encompass a spectrum of care settings, ranging from ambulatory surgical centers and dedicated dermatology clinics to hospital outpatient departments and specialty oncology practices.
Route of administration further refines therapeutic approaches, with oral Hedgehog inhibitors, intravenous immunotherapies, and topical formulations catering to variable disease stages and patient tolerability. Distribution channels play a pivotal role, as hospital pharmacies, retail pharmacies, and emerging online platforms each offer unique advantages in delivering medications and managing inventory logistics. Patient age group segmentation distinguishes cohorts under 45, those between 45 and 65, and populations over 65, reflecting divergent susceptibility patterns, comorbidity burdens, and treatment adherence considerations. Finally, classification by disease stage-early versus advanced BCC-guides treatment intensity, with early-stage lesions often amenable to local therapies and advanced presentations necessitating systemic or combination regimens. Understanding these interwoven segments is essential for stakeholders seeking to tailor strategies across the continuum of care.
This comprehensive research report categorizes the Basal Cell Carcinoma Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Drug Class
- Route Of Administration
- Patient Age Group
- Stage
- Distribution Channel
- End User
Analyzing key regional dynamics revealing how the Americas, EMEA, and Asia-Pacific each present unique opportunities and challenges in basal cell carcinoma management
Geographical dynamics exert a profound influence on BCC management and market evolution. In the Americas, particularly the United States, robust healthcare infrastructure, concentrated centers of dermatologic expertise, and high per-capita expenditure underpin rapid adoption of novel therapies and advanced diagnostic tools. The region has also been at the forefront of policy debates around drug pricing and import tariffs, which directly affect sourcing strategies and investment in local manufacturing facilities.
Within Europe, the Middle East, and Africa, diverse regulatory environments and reimbursement frameworks shape market access. European Union directives on pharmaceutical manufacturing standards and pharmacovigilance create a rigorous compliance landscape, while Middle Eastern and African markets exhibit growing demand for dermatologic services amid rising awareness and improving healthcare delivery. Concurrently, some governments are incentivizing domestic production to mitigate exposure to global trade disruptions.
In the Asia-Pacific region, expanding healthcare coverage, increasing patient awareness of skin cancer risks, and rising disposable incomes are driving greater demand for both surgical and non-surgical BCC treatments. However, disparities in resource availability and specialist access persist across emerging and established markets. Local manufacturers are increasingly engaging in public-private partnerships to enhance distribution networks and introduce cost-effective diagnostic platforms. Collectively, these regional nuances underscore the importance of tailored market entry and growth strategies to effectively meet evolving BCC care needs worldwide.
This comprehensive research report examines key regions that drive the evolution of the Basal Cell Carcinoma Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling the leading companies driving innovation in basal cell carcinoma therapies and diagnostics through strategic research and partnerships
A cohort of global biopharmaceutical leaders is shaping the future of BCC treatment through targeted R&D investments, strategic collaborations, and diversified product portfolios. Genentech, a subsidiary of Roche, pioneered hedgehog pathway inhibition with vismodegib, securing FDA approval in January 2012 based on durable response rates in advanced and metastatic BCC cohorts. Novartis entered the field in July 2015 with sonidegib, offering an alternative Hedgehog inhibitor whose pivotal trial demonstrated objective response rates above 50 percent in locally advanced disease.
Regeneron and Sanofi expanded the treatment paradigm by introducing Libtayo (cemiplimab-rwlc) in February 2021 as the first PD-1 inhibitor approved for advanced BCC, providing a critical option for patients who have progressed on or are intolerant to Hedgehog inhibitors. Concurrently, biomedical device companies are investing in advanced imaging systems such as reflectance confocal microscopy platforms, supporting earlier diagnosis and improved margin assessment.
Smaller biotechs and specialty dermatology enterprises are advancing photodynamic therapy innovations, novel topical formulations, and AI-enhanced diagnostic software. These diversified corporate strategies reflect an industry-wide commitment to addressing unmet needs across early-stage, advanced, and recurrent BCC patient populations.
This comprehensive research report delivers an in-depth overview of the principal market players in the Basal Cell Carcinoma Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Almirall, S.A.
- AstraZeneca PLC
- Bausch Health Companies Inc.
- Bayer AG
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences Inc.
- LEO Pharma A/S
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals Inc.
- Sanofi SA
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Verrica Pharmaceuticals Inc.
- Viatris Inc.
Strategic and actionable recommendations empowering industry leaders to enhance resilience and innovation in basal cell carcinoma treatment ecosystems
To navigate the evolving challenges in BCC treatment, industry leaders should prioritize supply chain diversification by increasing onshore API production capacity and establishing strategic partnerships with domestic contract manufacturers, thereby reducing exposure to tariff volatility and geopolitical risks. Additionally, investing in flexible manufacturing platforms capable of rapid technology transfers will enable swift scale-up of emerging therapies and support responsive inventory management.
Stakeholders are advised to enhance collaborative R&D initiatives that integrate immunotherapeutic agents with targeted inhibitors, exploring combination regimens backed by biomarker-driven patient selection to maximize clinical benefit. Engaging with regulatory authorities early in the development process can streamline approval pathways, particularly for novel modalities and diagnostic adjuncts. Moreover, expanding telehealth and digital monitoring solutions will improve patient engagement, adherence, and remote triage, thereby optimizing resource utilization.
Finally, forging alliances with academic centers and dermatology societies will facilitate real-world evidence generation, inform health-economic models, and strengthen reimbursement dossiers. By adopting these strategic imperatives, organizations can bolster resilience, drive innovation, and shape patient-centric care models in basal cell carcinoma treatment.
Meticulous research methodology detailing data sources, analytical frameworks, and validation techniques employed in basal cell carcinoma market analysis
This executive summary is underpinned by a comprehensive research methodology encompassing both secondary and primary data sources. Peer-reviewed clinical literature, regulatory filings, and company press releases were systematically reviewed to capture the latest developments in BCC therapeutics, diagnostics, and policy changes. Publicly available government and trade data provided insight into tariff structures, import-export dynamics, and manufacturing trends. Clinical trial registries and conference proceedings were analyzed to identify emerging investigational therapies and innovative diagnostic technologies.
To validate findings and enrich market context, expert interviews were conducted with key opinion leaders in dermatology, oncology, health economics, and supply chain management. Quantitative data triangulation and qualitative thematic analysis ensured accuracy and coherence across multiple dimensions, including treatment efficacy, market access, and competitive landscape. Segmentation frameworks were rigorously applied to delineate patient cohorts, care settings, and distribution channels. This methodology delivers a robust foundation for strategic decision making in the basal cell carcinoma treatment domain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Basal Cell Carcinoma Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Basal Cell Carcinoma Treatment Market, by Treatment Type
- Basal Cell Carcinoma Treatment Market, by Drug Class
- Basal Cell Carcinoma Treatment Market, by Route Of Administration
- Basal Cell Carcinoma Treatment Market, by Patient Age Group
- Basal Cell Carcinoma Treatment Market, by Stage
- Basal Cell Carcinoma Treatment Market, by Distribution Channel
- Basal Cell Carcinoma Treatment Market, by End User
- Basal Cell Carcinoma Treatment Market, by Region
- Basal Cell Carcinoma Treatment Market, by Group
- Basal Cell Carcinoma Treatment Market, by Country
- United States Basal Cell Carcinoma Treatment Market
- China Basal Cell Carcinoma Treatment Market
- Competitive Landscape
- List of Figures [Total: 19]
- List of Tables [Total: 1749 ]
Concluding reflections on the comprehensive executive summary and the imperative pathways for advancing basal cell carcinoma therapeutic innovation
In summary, the basal cell carcinoma treatment landscape is characterized by rapid innovation, shifting market dynamics, and evolving patient needs. The advent of immunotherapeutics such as PD-1 inhibitors, coupled with targeted pathway antagonists and advancements in noninvasive diagnostics, offers unprecedented opportunities to improve outcomes. Simultaneously, policy shifts and tariff measures underscore the critical importance of resilient supply chain strategies and cost-effective manufacturing solutions.
By integrating granular segmentation insights with regional and company-level analyses, stakeholders can tailor approaches that address distinct market imperatives and patient populations. Strategic investments in combination therapies, digital health, and domestic production capacity will be key to sustaining growth and enhancing patient access. The recommendations outlined herein provide a roadmap for industry leaders to navigate complexities, capitalize on emerging trends, and deliver impactful, patient-centric solutions.
Continued collaboration among pharmaceutical companies, device innovators, healthcare providers, and regulatory bodies will be essential to translating scientific breakthroughs into scalable clinical practices. The imperative now is to align resources, expertise, and vision toward a shared goal of elevating the standard of care for individuals affected by basal cell carcinoma.
Compelling call-to-action urging decision makers to engage directly with our Associate Director for specialized basal cell carcinoma market insights and report acquisition
I invite you to connect directly with Ketan Rohom, Associate Director of Sales & Marketing at 360iResearch, to explore tailored strategic insights and secure your comprehensive market research report on basal cell carcinoma treatments. Engaging with Ketan will empower your organization with authoritative guidance and actionable intelligence needed to make informed investment and product development decisions. Reach out today to unlock exclusive data access, gain a competitive edge, and accelerate your path to success in the rapidly evolving oncology landscape.

- How big is the Basal Cell Carcinoma Treatment Market?
- What is the Basal Cell Carcinoma Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




