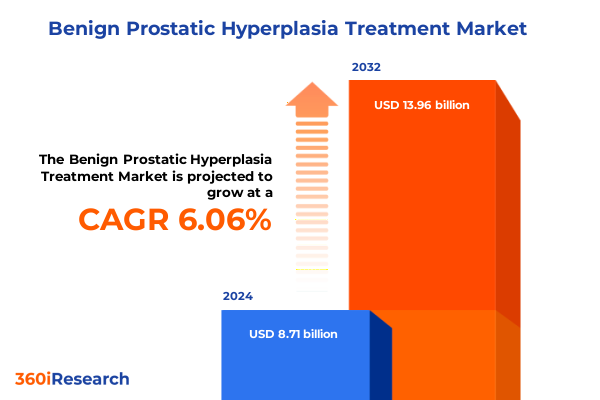
The Benign Prostatic Hyperplasia Treatment Market size was estimated at USD 9.20 billion in 2025 and expected to reach USD 9.74 billion in 2026, at a CAGR of 6.12% to reach USD 13.96 billion by 2032.
Navigating the Shifting Terrain of Benign Prostatic Hyperplasia Treatments Through Advances in Therapy Modalities and Market Transformation Trends
The benign prostatic hyperplasia treatment landscape is undergoing a profound transformation driven by demographic shifts, evolving patient expectations, and rapid technological advancements. As the global population ages, the prevalence of BPH continues to rise, creating a pressing need for therapies that balance efficacy with safety and patient quality of life. Historically dominated by pharmacological approaches, the market now encompasses an expanding arsenal of minimally invasive and surgical interventions that offer new pathways to symptom relief and disease management.
In recent years, emerging therapies have captured the attention of clinicians and patients alike. Iterations of alpha blockers and 5-alpha-reductase inhibitors have refined pharmacological profiles to enhance tolerability, while innovations such as water vapor therapy and prostate artery embolization are redefining the minimally invasive segment. Simultaneously, advances in laser surgery, prostatic urethral lift techniques, and refinements in traditional transurethral procedures are reshaping surgical treatment paradigms. These developments underscore a broader shift toward personalized medicine, where treatment decisions are increasingly informed by patient preferences, comorbidities, and health economics considerations.
Amid this convergence of demographic pressures and technological breakthroughs, stakeholders across the value chain-ranging from medical device manufacturers to healthcare providers-are recalibrating strategies to navigate complex regulatory, reimbursement, and competitive dynamics. By examining the core drivers of change and spotlighting key innovations, this executive summary provides a foundational overview for decision-makers seeking to harness opportunities in the evolving BPH treatment ecosystem.
Exploring Groundbreaking Shifts in BPH Treatment Paradigms as Technological Innovations and Regulatory Forces Reshape Patient Care Pathways
Over the past decade, the benign prostatic hyperplasia treatment ecosystem has witnessed a series of transformative shifts that are redefining care delivery and market dynamics. Technological innovation has accelerated the adoption of novel minimally invasive therapies, enabling outpatient interventions that reduce recovery times and lower overall cost burdens on healthcare systems. At the same time, iterative refinements in pharmacotherapy have improved the side effect profiles of traditional medications, enhancing patient adherence and long-term outcomes.
Regulatory agencies have played a pivotal role in this evolution, granting expedited pathways and real-world evidence frameworks to bring promising therapies to market more swiftly. Concurrently, health technology assessments and value-based procurement models are reshaping reimbursement structures, compelling manufacturers to demonstrate clear clinical and economic benefits. These policy and payment reforms are catalyzing collaborations between device companies and payers to develop comprehensive care bundles, further integrating surgical, minimally invasive, and pharmacological modalities.
Digital health solutions, including telemedicine platforms and remote monitoring tools, have also emerged as key enablers in BPH management. Virtual consultations streamline the diagnostic process and facilitate shared decision-making, while wearable sensors and mobile applications support post-procedural follow-up and symptom tracking. As patient-centric care models continue to gain traction, these digital innovations are set to become integral components of the BPH treatment journey, linking diagnostics, therapy, and long-term management in a cohesive continuum.
Assessing the Far-Reaching Consequences of Recent United States Trade Tariffs on the Supply Chain and Cost Structures of BPH Treatment Solutions
In early April 2025, the United States government introduced a broad suite of tariffs targeting imported medical devices and pharmaceutical inputs, imposing a 10% levy on certain Chinese-sourced products and extending derivative steel and aluminum duties of up to 25% on devices containing these metals. For benign prostatic hyperplasia treatments, this action has exerted significant pressure on cost structures, particularly for components such as urology laser systems, resectoscopes, electrodes, and catheters that rely heavily on global supply chains of specialized alloys and electronic assemblies.
Healthcare providers and hospital networks have reported increases in procurement expenses, prompting facility leaders to reevaluate supplier contracts and inventory strategies. The American Hospital Association has formally petitioned for exemptions on critical medical products, warning that sustained tariffs could disrupt patient care and lead to delays in accessing essential therapies such as minimally invasive water vapor treatments and prostatic urethral lift procedures. Furthermore, device manufacturers are accelerating efforts to diversify manufacturing footprints, exploring nearshoring opportunities in Mexico and reshoring initiatives within the United States to mitigate future trade policy volatility.
Despite the immediate cost headwinds, the tariff landscape has spurred a broader strategic pivot toward domestic innovation and supply chain resilience. Leading companies are forging partnerships with regional contract manufacturers and investing in advanced production technologies, including additive manufacturing for metal components and automated assembly lines for electronic modules. Over the longer term, these shifts may catalyze a reconfiguration of the BPH device supply chain, offering opportunities for stakeholders willing to invest in localized capabilities and collaborative sourcing frameworks.
Illuminating Critical Market Divides Across BPH Treatment Types, Product Portfolios, End Users, and Distribution Channels to Uncover Growth Levers
A nuanced examination of the benign prostatic hyperplasia treatment space reveals four critical segmentation axes that shape market dynamics and stakeholder strategies. When one considers treatment type, the market divides into pharmacological approaches, where generics and branded alpha blockers and 5-alpha-reductase inhibitors remain foundational; minimally invasive therapies, where techniques such as prostate artery embolization and water vapor therapy are gaining rapid traction; and surgical treatments, which span laser-based interventions, prostatic urethral lift, transurethral incision, and transurethral resection procedures and continue to serve as the gold standard for advanced cases.
From a product perspective, essential device categories underpinning these treatment types include specialized catheters for diagnostic and therapeutic access; electrodes employed in tissue ablation and coagulation; implants and prostatic stents that deliver sustained anatomical relief; resectoscopes facilitating endoscopic removal of obstructive tissue; and advanced urology lasers offering precise tissue vaporization. Each of these product clusters faces distinct competitive pressures, technological cycles, and regulatory requirements, informing tailored investment and product development trajectories.
End user segmentation further differentiates the landscape, as ambulatory surgical centers increasingly capture procedures traditionally performed in hospitals, while clinics and homecare settings leverage portable devices to extend the reach of pharmacotherapy support and non-invasive interventions. Concurrently, hospitals sustain their role as referral centers for high-complexity cases and acute management. Distribution channels overlay these end user dynamics, with offline channels retaining dominance for high-touch surgical technologies and consumables, even as online direct-to-provider and hybrid e-commerce models gain share by streamlining access to pharmaceuticals and single-use devices.
This comprehensive research report categorizes the Benign Prostatic Hyperplasia Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Product Type
- End User
- Distribution Channel
Revealing Distinct Regional Dynamics Across the Americas, Europe Middle East & Africa, and Asia Pacific That Shape BPH Treatment Adoption and Delivery
The Americas region continues to lead in benign prostatic hyperplasia treatment adoption, supported by established reimbursement policies and a high density of specialized care providers. Innovations in minimally invasive therapies are rapidly integrated into clinical guidelines, while hospitals and ambulatory surgical centers invest in advanced laser platforms and procedural suites. At the same time, digital health platforms are enabling remote patient monitoring, enhancing post-procedure follow-up and optimizing resource utilization across the continuum of care.
In Europe, the Middle East, and Africa, heterogeneous regulatory and reimbursement frameworks influence the pace of innovation uptake. Western European markets benefit from cohesive health technology assessment processes and cross-border harmonization, facilitating broader access to novel interventions like water vapor therapy. In contrast, regions within Eastern Europe, the Middle East, and Africa face infrastructure constraints and budgetary pressures, prioritizing cost-effective pharmacological management and incremental upgrades to existing endoscopic capabilities.
Asia-Pacific markets are characterized by rapid demographic shifts and expanding healthcare infrastructure. Countries such as China, India, and Australia are witnessing significant investments in state-of-the-art medical facilities, driving demand for next-generation surgical systems and minimally invasive therapies. However, cost sensitivity and local manufacturing policies are encouraging regional players to develop affordable device alternatives and generic pharmacotherapies, fostering a competitive environment that balances innovation with accessibility.
This comprehensive research report examines key regions that drive the evolution of the Benign Prostatic Hyperplasia Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Uncovering Strategic Initiatives and Competitive Strengths of Leading BPH Treatment Providers Driving Innovation and Market Leadership
Leading stakeholders in the benign prostatic hyperplasia treatment market are leveraging diversified portfolios and targeted innovations to strengthen their competitive positions. One prominent device manufacturer has focused on expanding the portfolio of minimally invasive therapies, notably investing in water vapor technology platforms and securing key regulatory approvals globally. Another key player has augmented its surgical offerings with next-generation laser systems, enhancing energy precision and ergonomic design to improve procedural outcomes.
Pharmaceutical incumbents continue to refine alpha blocker and 5-alpha-reductase inhibitor formulations, emphasizing sustained-release profiles and reduced adverse events to bolster patient adherence. At the same time, strategic acquisitions have enabled companies to integrate complementary device technologies and broaden geographic reach. Collaborations between medical technology firms and contract research organizations have accelerated clinical evidence generation, supporting expanded label claims and reimbursement approvals in priority markets.
Emerging challengers and regional innovators are also reshaping competitive dynamics by developing cost-effective device variants and localized manufacturing capabilities. These entrants are achieving traction in price-sensitive markets while entering into partnerships with global distributors to scale their offerings. Across the board, the orchestration of product innovation, regulatory strategy, and commercial execution is defining the leadership contours of the rapidly evolving BPH treatment landscape.
This comprehensive research report delivers an in-depth overview of the principal market players in the Benign Prostatic Hyperplasia Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Alembic Pharmaceuticals Limited
- Asahi Kasei Corporation
- Astellas Pharma Inc.
- Bayer AG
- Boehringer Ingelheim GmbH
- Boston Scientific Corporation
- Cipla Ltd.
- Coloplast Group
- Dr. Reddy’s Laboratories
- Eli Lilly and Company
- Endo International PLC
- GlaxoSmithKline plc
- Hikma Pharmaceuticals PLC
- IPG Photonics Corporation
- Lumenis Ltd.
- Olympus Corporation
- Organon group of companies
- PROCEPT BioRobotics Corporation
- ProstaLund AB
- Quanta System
- Richard Wolf GmbH
- Teleflex Incorporated
- Urologix, LLC.
Crafting Practical Strategic Imperatives for BPH Treatment Stakeholders to Capitalize on Emerging Opportunities and Mitigate Industry Challenges
Industry leaders are advised to prioritize investments in next-generation minimally invasive technologies that align with value-based care models and evolving reimbursement frameworks. By showcasing clear clinical and economic benefits through robust real-world evidence, organizations can secure favorable coverage and drive rapid adoption of therapies such as water vapor ablation and prostatic artery embolization.
Simultaneously, manufacturers should accelerate supply chain diversification to mitigate the impacts of trade policy volatility and tariff pressures. Establishing strategic partnerships with regional contract manufacturers and exploring onshore production of critical components will enhance resilience and support more predictable cost structures.
Stakeholders must also cultivate collaborative relationships with regulatory bodies and health technology assessment agencies to streamline approval pathways and secure early market access. Developing comprehensive health economics dossiers and engaging in multi-stakeholder advisory panels can substantiate the value proposition of innovative treatments and inform policy decisions.
Finally, embracing digital health and omnichannel engagement strategies will strengthen customer relationships and extend care beyond traditional settings. Integrating telemedicine for patient screening, leveraging mobile applications for symptom tracking, and deploying remote diagnostic tools can differentiate offerings and unlock new channels for therapy initiation and follow-up.
Detailing a Robust Research Framework Employing Multi-Source Data Acquisition and Rigorous Analysis to Secure Actionable Market Intelligence
This research employed a comprehensive, multi-tiered approach to ensure the reliability and validity of findings. Secondary research encompassed extensive review of peer-reviewed clinical studies, regulatory filings from global health authorities, and policy analyses from trade associations to establish a robust contextual framework. Industry publications, conference proceedings, and company press releases supplemented these sources with timely insights into product launches and competitive developments.
Primary research included in-depth interviews with key opinion leaders, interventional urologists, procurement specialists, and health system administrators across major geographic markets. These qualitative engagements provided first-hand perspectives on clinical adoption drivers, technology preferences, and barriers to market entry. Survey data from healthcare providers and supply chain executives further quantified sentiment around trade policy impacts and future investment priorities.
Data triangulation methodologies were applied to reconcile discrepancies and validate trends, employing both top-down and bottom-up analytical techniques. A structured codebook guided the classification of treatment types, product categories, end users, and distribution channels to generate consistent segmentation insights. Tariff and trade policy analysis leveraged publicly available government documents, trade data, and industry commentary to assess the cumulative impact on supply chains and cost structures.
Finally, iterative review sessions with senior industry advisors and methodological experts ensured analytical rigor and strategic relevance. This framework underpins the actionable intelligence presented in this executive summary, delivering a clear, evidence-based foundation for decision-making in the benign prostatic hyperplasia treatment domain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Benign Prostatic Hyperplasia Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Benign Prostatic Hyperplasia Treatment Market, by Treatment Type
- Benign Prostatic Hyperplasia Treatment Market, by Product Type
- Benign Prostatic Hyperplasia Treatment Market, by End User
- Benign Prostatic Hyperplasia Treatment Market, by Distribution Channel
- Benign Prostatic Hyperplasia Treatment Market, by Region
- Benign Prostatic Hyperplasia Treatment Market, by Group
- Benign Prostatic Hyperplasia Treatment Market, by Country
- United States Benign Prostatic Hyperplasia Treatment Market
- China Benign Prostatic Hyperplasia Treatment Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1272 ]
Synthesizing Core Findings and Strategic Implications to Illuminate the Future Trajectory of BPH Treatment Innovations and Market Evolution
The benign prostatic hyperplasia treatment landscape stands at a critical inflection point where demographic pressures, technological innovations, and policy drivers converge to reshape care paradigms. The evolution from traditional pharmacotherapy toward a more diversified mix of minimally invasive and surgical solutions reflects the broader shift toward personalized, value-based care. Meanwhile, the imposition of trade tariffs has underscored the strategic importance of supply chain resilience and domestic innovation.
Segmentation insights reveal differentiated growth trajectories across treatment types, product categories, end users, and distribution channels, highlighting the need for tailored strategies that address distinct clinical and commercial dynamics. Regionally, the Americas, EMEA, and Asia-Pacific each present unique opportunities and challenges, driven by reimbursement environments, regulatory frameworks, and healthcare infrastructure investments.
Competitive analysis underscores the role of strategic partnerships, regulatory expertise, and differentiated product portfolios in defining market leadership. As industry stakeholders chart pathways forward, the imperative to demonstrate robust clinical evidence, optimize cost structures, and engage in collaborative policy dialogues will be key to capturing emerging opportunities.
Collectively, the insights and recommendations outlined herein provide a comprehensive roadmap for navigating the complexity of the BPH treatment market. By aligning innovation, operational resilience, and customer-centric engagement, organizations can position themselves to deliver superior patient outcomes and sustainable growth in this dynamic therapeutic arena.
Unlock Exclusive BPH Treatment Market Intelligence by Connecting with Our Associate Director to Drive Your Strategic Decisions
Ready to elevate your strategic planning with unparalleled insights into the benign prostatic hyperplasia treatment market? Connect directly with Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch, to secure your copy of the full market research report today and gain actionable intelligence to inform your next move.

- How big is the Benign Prostatic Hyperplasia Treatment Market?
- What is the Benign Prostatic Hyperplasia Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




