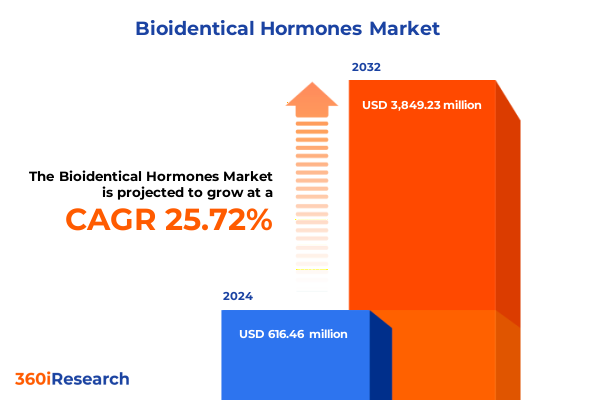
The Bioidentical Hormones Market size was estimated at USD 773.91 million in 2025 and expected to reach USD 962.86 million in 2026, at a CAGR of 25.75% to reach USD 3,849.23 million by 2032.
Embracing Personalized Hormonal Care Transforming Patient Outcomes Through Innovative Bioidentical Hormone Therapies and Evolving Industry Practices
The realm of bioidentical hormone therapy has undergone a profound transformation driven by an increasing emphasis on personalized patient care and a deeper understanding of endocrine health. As healthcare providers move toward tailor-made treatment regimens, bioidentical formulations that replicate native hormone structures have emerged as a pivotal option for patients seeking symptom relief with a more natural profile. This shift reflects broader trends in precision medicine where individual patient characteristics-from genetic profiles to lifestyle factors-inform therapeutic decisions and optimize outcomes.
Moreover, patient awareness and demand for alternatives to traditional synthetic hormones have surged in recent years. Consumers are now more empowered than ever to research treatment options, engage in shared decision-making with their clinicians, and voice preferences for therapies perceived as safer or more compatible with their physiology. In response, compounding pharmacies and pharmaceutical manufacturers alike have expanded their portfolios to include a diverse array of bioidentical hormone preparations, ranging from topical gels to oral capsules, each designed to meet specific patient needs.
In parallel, evolving regulatory frameworks are reshaping industry practices by promoting enhanced quality standards, rigorous testing protocols, and transparent labeling. These developments underscore the critical importance of reliability and consistency in hormone therapies, ensuring that patients and providers can trust that each dose delivers the intended therapeutic effect without unexpected variability. As we embark on this executive summary, it is essential to recognize that the stage is set for continued innovation and dynamic growth as stakeholders across the value chain collaborate to advance personalized endocrine care.
Navigating Major Transformative Shifts Redefining Bioidentical Hormone Therapy Through Regulatory Advances Patient-Centric Models and Technological Breakthroughs
The bioidentical hormone landscape has been reshaped by a confluence of technological breakthroughs and shifting regulatory expectations. Foremost among these is the advent of advanced compounding processes that employ precision dosage techniques, such as microfluidics and three-dimensional printing, to ensure uniformity in each delivery form. These innovations have accelerated the development of products that cater to highly specific patient needs, enabling clinicians to fine-tune hormone ratios and concentrations with unprecedented accuracy.
Furthermore, the rapid proliferation of telemedicine and digital health platforms has revolutionized patient engagement and continuity of care. Virtual consultation models facilitate ongoing monitoring of hormonal status through remote biometric data collection, empowering patients to adjust therapy under clinical supervision without frequent in-office visits. Such connectivity not only enhances adherence but also provides real-time insights that inform iterative treatment modifications.
On the regulatory front, recent guidance initiatives have introduced stringent requirements for compounding pharmacies, reinforcing expectations around ingredient sourcing, stability testing, and batch traceability. These measures aim to elevate product safety and reinforce public confidence in compounded bioidentical hormone therapies. Simultaneously, payer policies are increasingly recognizing the long-term value of personalized hormone care, prompting the integration of outcome-based reimbursement models that reward demonstrable improvements in patient quality of life.
As a result of these transformative trends, the industry is experiencing a paradigm shift toward data-driven, patient-centric approaches. Stakeholders must therefore remain vigilant, embracing new technologies and regulatory mandates as catalysts for growth rather than impediments to innovation.
Assessing the Cumulative Impact of United States Tariffs in 2025 on Bioidentical Hormone Ingredient Supply Chains Cost Structures and Market Dynamics
The implementation of new tariffs on key hormone precursors and excipients effective in early 2025 has introduced a fresh set of challenges to the bioidentical hormone sector. Suppliers sourcing raw materials from traditional low-cost manufacturing hubs encountered increased import duties, directly affecting the landed cost of active pharmaceutical ingredients. Consequently, compounding pharmacies and formulation specialists have had to reassess their procurement strategies to mitigate margin erosion and preserve competitive pricing structures.
In response, many stakeholders accelerated initiatives to diversify their supply chains, forging relationships with domestic and regional producers that could offer greater logistical resilience. Although these alternative sources often carry a modest cost premium, the payoff in reduced lead times and diminished exposure to tariff volatility has proven substantial. This strategic pivot has also stimulated nearshoring investments, encouraging local manufacturers to scale up capabilities for steroid hormone synthesis under stringent quality certifications.
Moreover, the pass-through of incremental cost increases has varied across delivery formats and end-user channels. Healthcare facilities operating under bundled payment arrangements have been particularly sensitive to formulary cost pressures, while direct-to-consumer compounding services have leveraged transparent pricing models to justify modest price adjustments. In certain instances, strategic partnerships between ingredient suppliers and pharmacy networks have facilitated cost-sharing agreements that cushion the financial impact on patients.
Ultimately, the cumulative influence of tariff adjustments in 2025 has galvanized the industry toward greater supply chain agility and closer collaboration among raw material vendors, compounding entities, and distribution partners. By embracing these adaptive measures, market participants are positioning themselves to navigate ongoing trade policy fluctuations with greater confidence.
Uncovering Key Segmentation Insights in Bioidentical Hormone Therapy Across Products End Users Therapies Delivery Methods Age and Gender Demographics
An in-depth examination of product type reveals that topically applied formulations maintain a strong foothold, with creams continuing to serve as a preferred vehicle for gradual hormone absorption. Meanwhile, gels and sprays are gaining traction among patient cohorts seeking rapid onset and ease of administration. Tablets remain a mainstay for oral dosing, valued for their convenience and precise quantification of hormone content.
When considering end-user segments, specialized clinics retain a leadership position by offering holistic patient management programs that integrate diagnostic testing, therapeutic oversight, and wellness education. Home care services have emerged as a dynamic channel, especially for aging populations who prioritize comfort and privacy. Hospitals continue to rely on established compounding pharmacies to support inpatient protocols, whereas retail pharmacies offer over-the-counter access for stabilized dose regimens, reinforcing the continuum of care from clinical settings to personal environments.
Therapy type breakdown underscores the enduring prominence of estrogen preparations among female patients experiencing menopausal symptoms. Progesterone formulations play a critical supporting role in balancing estrogen effects and promoting endometrial health, while testosterone therapies have witnessed robust uptake among men confronting age-related hormonal decline. The intersection of gender and age group analysis highlights that women aged fifty to sixty-four represent the largest cohort of estrogen and progesterone users, whereas testosterone administration is most prevalent among males aged sixty-five and older.
Observations around delivery method further emphasize the versatility of injectable options, which cater to patients requiring high-concentration dosing under medical supervision. Oral capsules offer discrete and familiar dosing formats, complemented by tablets that excel in stability. Topical vehicles-spanning creams gels and sprays-underscore the importance of customizable absorption profiles and underscore the sector’s commitment to patient preference and adherence across diverse demographic segments.
This comprehensive research report categorizes the Bioidentical Hormones market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Therapy Type
- Delivery Method
- Age Group
- Gender
- End User
Analyzing Regional Dynamics Shaping the Bioidentical Hormone Market Landscape Across Americas Europe Middle East Africa and Asia-Pacific
In the Americas, the United States and Canada lead a mature market characterized by a dense network of compounding pharmacies and specialized wellness centers. Regulatory frameworks emphasize strict quality standards, and reimbursement models have gradually begun to incorporate value-based elements that prioritize patient-reported outcomes. Consumer awareness campaigns and professional education initiatives have further cemented the region’s status as a global innovator in bioidentical hormone applications.
Across Europe the Middle East and Africa, evolving guidance from supranational health authorities has prompted harmonization efforts aimed at standardizing compounding practices and enforcing transparent labeling. Variability in national reimbursement schemes persists, with certain Western European countries offering coverage for hormone therapies under defined clinical criteria. Meanwhile emerging markets in the Middle East and parts of Africa are experiencing a nascent rise in demand, driven by urbanization and increasing healthcare infrastructure investment.
The Asia-Pacific region presents a heterogeneous landscape. Australia and Japan exhibit well-established regulatory ecosystems that support both pharmaceutical and compounded bioidentical therapies, while rapid demographic aging in countries such as South Korea fuels growing demand for menopause and andropause treatments. In contrast, stringent local manufacturing requirements and evolving import regulations in China and India present entry barriers for global suppliers, even as rising health awareness creates opportunities for market entrants willing to navigate complex approval pathways.
Taken together, these regional insights illuminate the necessity of tailored market entry strategies and partnership models that align with local regulatory regimes, reimbursement dynamics, and patient care preferences.
This comprehensive research report examines key regions that drive the evolution of the Bioidentical Hormones market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Strategic Moves and Innovations from Leading Companies Propelling Growth and Advancements in the Bioidentical Hormone Sector
A number of specialized compounding pharmacies have distinguished themselves through investments in proprietary formulation platforms and robust quality assurance protocols. These organizations have expanded their service offerings to include telehealth consultations and home-delivery programs, reinforcing their value proposition in an increasingly competitive environment. Several of these pharmacies have also pursued strategic alliances with raw material suppliers to secure priority access to high-purity hormone precursors.
On the pharmaceutical front, established players have augmented their pipelines with bioidentical variants of traditional hormone products. Through acquisitions and licensing agreements, they have incorporated smaller niche manufacturers into their portfolios, thereby enhancing their capacity to deliver both mass-market and personalized solutions. This dual approach enables these companies to leverage existing distribution networks while catering to high-margin variables within the compounding space.
Digital health startups are also making inroads by partnering with clinics to integrate treatment monitoring platforms and mobile applications that track symptom relief and biomarker trends. By blending data analytics with patient engagement tools, these ventures are contributing to a more iterative and evidence-based approach to hormone therapy management. Furthermore, a number of laboratory service providers have launched specialized testing panels for hormone metabolites, thereby deepening the feedback loops that inform dosage adjustments.
Collectively, these strategic moves underscore a broader trend toward collaboration across the value chain. Whether through co-development agreements, distribution partnerships or technology integrations, leading organizations are aligning capabilities to address evolving patient expectations and regulatory demands in the bioidentical hormone sector.
This comprehensive research report delivers an in-depth overview of the principal market players in the Bioidentical Hormones market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Actiza Pharmaceutical Private Limited
- Bayer AG
- BioTE Medical LLC
- Defy Medical
- Full Life Wellness Center
- GEA Group Aktiengesellschaft
- Gedeon Richter PLC
- GeneScience Pharmaceuticals Co Ltd
- Merck KGaA
- Mithra Pharmaceuticals S.A.
- Novartis AG
- Noven Pharmaceuticals Inc
- Novo Nordisk A/S
- Orion Corporation
- Papillon Medical
- Pfizer Inc
- SottoPelle
- Teva Pharmaceutical Industries Ltd
- TherapeuticsMD Inc
- Viatris Inc
- ZRT Laboratory
Empowering Industry Leaders with Actionable Recommendations to Navigate Regulatory Complexities Enhance Patient Engagement and Accelerate Innovation
To thrive in the rapidly evolving bioidentical hormone arena organizations should prioritize the establishment of rigorous quality management systems that align with the latest compounding and pharmaceutical standards. By investing proactively in real-time analytics for batch verification and stability testing, stakeholders can not only ensure compliance but also cultivate trust among patients and prescribers. Embracing automated monitoring tools and digital batch records will further enhance operational transparency.
Simultaneously, integrating telemedicine platforms with personalized dosing algorithms can strengthen patient adherence and streamline clinical workflows. Through strategic partnerships with digital health providers, companies can offer end-to-end solutions that encompass remote diagnostics, virtual follow-ups, and adaptive therapy adjustments. This integrated model will bolster patient satisfaction and position organizations as forward-thinking leaders in hormone care.
In light of ongoing trade policy fluctuations, stakeholder collaboration on supply chain diversification is paramount. Engaging in joint forecasting arrangements with ingredient suppliers, exploring nearshoring opportunities, and negotiating flexible cost-sharing agreements will mitigate risk and support stable access to essential APIs. Concurrently, active participation in industry advocacy groups can inform policy decisions and protect the integrity of compounding practices.
Finally, fostering educational initiatives for clinicians and end users will help demystify bioidentical therapies and underscore their clinical merits. Tailored training programs, peer-to-peer knowledge exchanges, and evidence-based outreach campaigns can drive broader acceptance and optimize therapeutic outcomes. Together these recommendations form a cohesive roadmap for organizations aiming to secure sustainable growth and leadership in bioidentical hormone therapy.
Detailing the Rigorous Research Methodology Underpinning Bioidentical Hormone Analysis Through Systematic Data Collection Validation and Expert Consultation
The foundation of this analysis rests on a multi-phase research methodology designed to ensure both breadth and depth of insight. Initial secondary research included a systematic review of peer-reviewed scientific journals, regulatory agency publications, and professional society guidelines to map the evolving standards and clinical evidence supporting bioidentical hormone therapies.
Subsequent primary research involved structured interviews with a diverse cross-section of stakeholders, including endocrinologists, compounding pharmacists, regulatory affairs specialists, and distribution channel executives. These interviews provided qualitative perspectives on market drivers, operational challenges, and emerging best practices. In addition, a quantitative survey was administered to a representative sample of clinics and home care providers to capture usage patterns and procurement preferences.
Data triangulation was achieved by cross-referencing insights from secondary sources with primary inputs and validated through consultations with an expert advisory panel comprising leading academic researchers and clinical practitioners. Advanced analytics techniques, including cluster analysis and regression modeling, were employed to identify significant correlations between therapy attributes and patient outcomes. Rigorous data cleansing protocols and standardized coding frameworks were applied to maintain consistency and reliability across multiple datasets.
Finally, all findings underwent a comprehensive peer review process to verify factual accuracy and interpretive validity. This methodological rigor ensures that the insights presented in this report reflect both the current state and future trajectory of the bioidentical hormone market with a high degree of confidence.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Bioidentical Hormones market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Bioidentical Hormones Market, by Product Type
- Bioidentical Hormones Market, by Therapy Type
- Bioidentical Hormones Market, by Delivery Method
- Bioidentical Hormones Market, by Age Group
- Bioidentical Hormones Market, by Gender
- Bioidentical Hormones Market, by End User
- Bioidentical Hormones Market, by Region
- Bioidentical Hormones Market, by Group
- Bioidentical Hormones Market, by Country
- United States Bioidentical Hormones Market
- China Bioidentical Hormones Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1431 ]
Synthesizing Key Findings and Future Outlook to Illuminate Strategic Imperatives and Growth Pathways in the Evolving Bioidentical Hormone Landscape
In synthesizing the findings of this executive summary it becomes clear that the bioidentical hormone landscape is poised for continued evolution driven by personalized care imperatives, technological advancements, and regulatory refinements. The interplay of innovative delivery platforms, expanding telehealth modalities, and diversified supply chain strategies has established a more resilient and patient-centric ecosystem than ever before.
Segmentation insights reveal nuanced preferences across product types and end-user channels, highlighting opportunities for differentiated value propositions in topical, oral, and injectable formats. Regional variances underscore the importance of tailored market approaches, particularly in navigating the unique regulatory and reimbursement environments of the Americas, Europe Middle East Africa, and Asia-Pacific. Corporate strategies that harmonize scale advantages with niche compounding capabilities are emerging as a competitive hallmark.
As tariffs and trade policies continue to shape cost dynamics, organizations that proactively engage in supply chain diversification and policy advocacy will be best positioned to safeguard margins and ensure uninterrupted patient access. Moreover, the convergence of real-world evidence platforms with patient engagement tools offers a powerful framework for demonstrating clinical value and securing favorable payer coverage.
Ultimately, the evolving bioidentical hormone sector demands a balanced focus on operational excellence, scientific rigor, and patient experience. By embracing these strategic imperatives today, industry participants can chart a path toward sustainable growth and transformative impact for the patients they serve.
Connect Directly with Ketan Rohom Associate Director Sales and Marketing to Secure Comprehensive Bioidentical Hormone Market Research Insights
To discover the full breadth of market dynamics, detailed segmentation analysis, and strategic imperatives in the bioidentical hormone space, you are invited to reach out directly to Ketan Rohom Associate Director Sales and Marketing for an exclusive consultation. Engaging with Ketan will provide you with personalized insights tailored to your organization’s unique strategic objectives and operational challenges. Through this conversation you will gain clarity on how to leverage emerging trends, navigate regulatory complexities, and optimize patient engagement models within your own portfolios. Secure your access to the comprehensive market research report today and empower your leadership team with the data and context needed to drive competitive advantage in an evolving therapeutic landscape. Connect directly with Ketan Rohom to initiate your purchase and begin charting the next phase of growth in bioidentical hormone therapy.

- How big is the Bioidentical Hormones Market?
- What is the Bioidentical Hormones Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




