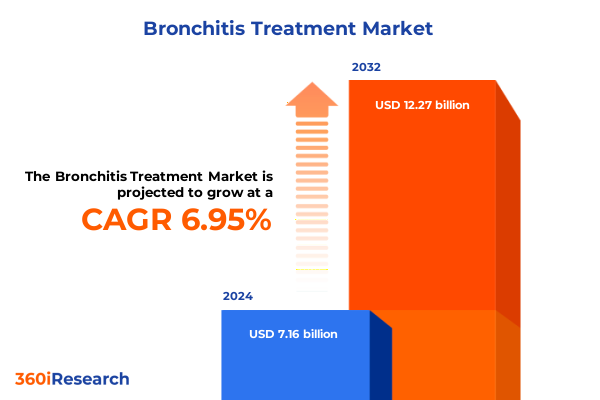
The Bronchitis Treatment Market size was estimated at USD 7.66 billion in 2025 and expected to reach USD 8.20 billion in 2026, at a CAGR of 6.95% to reach USD 12.27 billion by 2032.
Understanding the Complex Bronchitis Treatment Domain Amid Changing Patient Expectations Emerging Therapy Innovations and Healthcare Delivery Shifts
Bronchitis, characterized by inflammation of the bronchial tubes, presents in acute and chronic forms and poses significant clinical challenges worldwide. Acute bronchitis often results from viral infections leading to cough and mucus production, whereas chronic bronchitis-a subtype of chronic obstructive pulmonary disease-requires long-term management of persistent symptoms. In both scenarios, patient adherence, comorbidity management, and antimicrobial stewardship remain central considerations for healthcare providers. Recent shifts in diagnostic criteria and the increased use of point-of-care testing have further influenced treatment pathways and resource allocation within diverse healthcare settings.
This executive summary offers a comprehensive exploration of the current bronchitis treatment landscape, highlighting transformative trends, tariff impacts within the United States context, and critical segmentation and regional dynamics. It distills key insights into therapy types, drug classes, administration routes, distribution models, end users, demographic considerations, and product forms. Additionally, it outlines major industry players, actionable recommendations, and the robust research methodology underpinning these findings. Stakeholders will find a coherent synthesis of strategic imperatives designed to optimize clinical outcomes and drive operational excellence in bronchitis management. By synthesizing diverse data sources and expert perspectives, this summary sets the stage for informed decision making and strategic investment in bronchitis treatment innovations.
Identifying Pioneering Trends Reshaping Bronchitis Treatment Paradigms from Digital Diagnostics to Personalized Therapeutic Strategies
Across the bronchitis treatment ecosystem, innovations in digital diagnostics and telehealth platforms are rapidly reshaping clinical practice. Remote monitoring of respiratory function and integration of artificial intelligence in symptom tracking enable earlier intervention and continuous patient engagement. Healthcare systems are increasingly adopting teleconsultations to extend access to care, reduce hospital visits, and support chronic disease management. Concurrently, digital adherence tools and wearable devices facilitate real-time data collection, creating feedback loops that enhance therapy optimization and patient empowerment.
Meanwhile, therapeutic strategies are evolving toward precision and personalization, with novel combination inhalers and targeted drug delivery systems gaining prominence. Emphasis on antimicrobial stewardship has led to refined protocols that balance efficacy against the risks of resistance. Herbal supplements and complementary therapies are also being revisited through rigorous clinical studies, reflecting broader interest in integrative approaches. Moreover, collaboration between technology firms and pharmaceutical manufacturers is facilitating the co-development of smart delivery devices, pointing to an era where digital and pharmacological interventions converge seamlessly. As a result, stakeholder collaboration and data-driven insights are poised to redefine standards of care for bronchitis patients globally.
Analyzing the Cumulative Implications of Recent United States Tariff Measures on Bronchitis Treatment Supply Chains and Cost Structures
The implementation of new tariff measures in early 2025 has exerted tangible pressure on supply chains for bronchitis treatment components, particularly active pharmaceutical ingredients, key excipients, and specialized inhaler devices imported into the United States. Manufacturers report increased procurement costs and extended lead times, forcing procurement teams to reassess vendor relationships and contract terms. These measures have also indirectly influenced raw material availability, with certain international suppliers redirecting production toward markets less affected by trade barriers. As cost structures adjust, pricing pressures have surfaced across distribution channels, from hospital pharmacies to retail and online outlets.
In response, industry participants are accelerating efforts to diversify sourcing strategies and invest in domestic manufacturing capabilities. Some pharmaceutical companies are exploring partnerships with local API producers and repurposing existing facilities to mitigate dependency on affected imports. Meanwhile, supply chain managers are implementing dual sourcing models and contingency planning to preserve consistent product availability. Payers and providers are also adapting reimbursement protocols to account for evolving cost dynamics, ensuring patient access remains a priority. Moreover, research and development divisions have recalibrated budgeting forecasts to absorb tariff-induced expenses, recognizing that innovation pipelines may be affected if cost inflation is not addressed. Regulatory authorities have initiated dialogues with industry groups to facilitate expedited approvals for locally sourced alternatives, highlighting a collaborative approach to maintaining continuity in drug development. Through these adaptive strategies, stakeholders aim to transform tariff challenges into opportunities for reinforcing supply chain robustness and fostering long-term operational resilience.
Revealing Critical Segment Dynamics Driving Bronchitis Treatment Decisions across Therapy Types Drug Classes Routes of Administration and Patient Demographics
Key segmentation frameworks unveil how patient needs, clinical protocols, and channel infrastructures intersect. Evaluating bronchitis treatment options through the lens of therapy type reveals distinct decision drivers across herbal supplements, over-the-counter remedies, and prescription medications. Within prescription drugs, the classification of active agents further delineates market dynamics: antibiotics span categories such as cephalosporins, macrolides, penicillins, and tetracyclines, while bronchodilator choices pivot between anticholinergics and beta agonists. Corticosteroid interventions encompass inhaled, intravenous, and oral formulations, each catering to specific clinical scenarios. Meanwhile, expectorant strategies, primarily involving agents like bromhexine and guaifenesin, continue to be leveraged for symptom management and mucus clearance.
In parallel, routes of administration ranging from inhalation and oral dosing to topical applications shape patient adherence profiles and therapeutic outcomes. Distribution pathways including hospital pharmacies, online platforms, and retail outlets modify access points and influence channel-specific dynamics. End users span clinics, homecare environments, and hospitals, reflecting the broad spectrum of care settings engaged in bronchitis treatment. Demographic segmentation adds further nuance, with adult, geriatric, and pediatric populations exhibiting unique therapeutic requirements and safety considerations. Finally, product form preferences-whether capsules, inhalers, liquids, or tablets-intersect with patient lifestyle factors and administration convenience, guiding both prescribing decisions and consumer acceptance across diverse treatment scenarios. By integrating these segmentation insights, stakeholders can refine targeting strategies and design tailored interventions that resonate with specific patient cohorts and distribution contexts.
This comprehensive research report categorizes the Bronchitis Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Drug Class
- Treatment Type
- Route Of Administration
- Age Group
- Product Form
- End User
- Distribution Channel
Decoding Regional Variations in Bronchitis Treatment Adoption across Americas Europe Middle East Africa and Asia-Pacific Healthcare Ecosystems
Across the Americas, bronchitis treatment strategies are influenced by advanced healthcare infrastructures, insurance coverage variations, and evolving telehealth networks. In the United States and Canada, hospital and outpatient systems leverage digital platforms to support remote monitoring and prescription management, while partnerships between healthcare providers and community pharmacies enhance patient education on proper inhaler techniques and medication adherence. Latin American markets display growing interest in generic and cost-effective formulations, with public health initiatives often prioritizing access to essential respiratory medications in underserved regions.
In Europe, the Middle East, and Africa, regulatory harmonization under health agencies and regional blocs shapes drug approval timelines and quality standards, encouraging manufacturers to align with stringent compliance protocols. Many countries are also integrating antimicrobial stewardship frameworks into national treatment guidelines, thereby influencing antibiotic prescribing behaviors. Meanwhile, in the Asia-Pacific region, rapid modernization of healthcare delivery systems coexists with substantial reliance on traditional remedies and herbal supplements, particularly in markets where cultural preferences and cost considerations drive consumer choices. Cross-regional collaboration on clinical research and knowledge exchange has further accelerated, promoting global best practices in bronchitis management and highlighting the importance of context-sensitive approaches to treatment adoption. Moreover, demographic shifts and urbanization trends across these regions continue to redefine demand patterns, underscoring the necessity for agile strategies that accommodate diverse patient profiles and healthcare landscapes.
This comprehensive research report examines key regions that drive the evolution of the Bronchitis Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Mapping Leading Industry Players and Collaborative Ventures Shaping Innovation in Bronchitis Treatment Formulations and Delivery
Leading pharmaceutical companies have intensified R&D investments in novel bronchitis therapies, spanning next-generation bronchodilator molecules and long-acting combination inhalers. Collaboration with biotech firms has facilitated the discovery of targeted biologics aimed at modulating inflammatory pathways, while strategic alliances with academic institutions support advanced clinical trials in both acute and chronic bronchitis populations. Generic drug manufacturers are concurrently expanding their portfolios to offer cost-competitive antibiotic and expectorant alternatives, responding to healthcare payer pressures and growing demand for accessible treatment options.
Simultaneously, digital health enterprises and medical device developers are driving innovation in smart inhaler technologies, respiratory monitoring sensors, and mobile applications that enhance patient engagement and adherence tracking. These partnerships underscore a shift toward integrated care models, where pharmacological and digital solutions converge to deliver personalized treatment regimens. In addition, several multinational corporations are establishing regional manufacturing hubs to circumvent trade barriers and optimize product distribution. Furthermore, merger and acquisition activities, licensing agreements, and joint ventures are commonplace as companies seek to augment their treatment pipelines and leverage complementary expertise across geographies. Together, these corporate strategies reflect an ecosystem increasingly defined by collaborative innovation, cross-sector alliances, and a shared commitment to elevating bronchitis patient outcomes.
This comprehensive research report delivers an in-depth overview of the principal market players in the Bronchitis Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- American Well Corporation
- AstraZeneca PLC
- Boehringer Ingelheim International GmbH
- Cadila Pharmaceuticals Limited
- Cigna Corporation
- Cipla Limited
- Dr. Reddy’s Laboratories Ltd
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline Pharmaceuticals Limited
- Johnson & Johnson Services, Inc
- Lupin Limited
- Melinta Therapeutics, Inc.
- Merck and Co. Inc.
- Novartis International AG
- Pfizer Inc.
- Reckitt Benckiser Group PLC
- Sanofi S.A
- Sun Pharmaceutical Industries Ltd.
- TelaCare Health Solutions, LLC
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
Implementing Strategic Initiatives to Enhance Market Positioning and Patient Outcomes within the Bronchitis Treatment Ecosystem
Industry leaders should prioritize the integration of telemedicine services with traditional treatment pathways, ensuring that digital consultations and remote monitoring tools are seamlessly incorporated into standard clinical workflows. By deploying data analytics to interpret patient-reported outcomes and adherence metrics in real time, organizations can identify at-risk cohorts earlier and tailor interventions proactively. In parallel, establishing multidisciplinary antimicrobial stewardship committees will support the optimization of antibiotic utilization, reducing the incidence of resistance while maximizing therapeutic efficacy. Investment in clinician and patient education programs focused on proper inhaler techniques and symptom management protocols will further enhance treatment consistency and satisfaction.
Manufacturers and supply chain stakeholders are advised to diversify sourcing models by forging partnerships with domestic API producers and exploring alternative raw material suppliers to mitigate tariff-driven disruptions. Embracing flexible manufacturing frameworks, such as contract development and manufacturing organizations, can bolster production agility and responsiveness. Strategic collaborations with local healthcare providers and payers may also facilitate the design of value-based care agreements that align reimbursement structures with patient outcomes. Additionally, conducting local clinical studies and generating real-world evidence will empower stakeholders to adapt treatment protocols to regional patient characteristics and payer requirements, thereby strengthening market access and uptake. Ultimately, a proactive, cross-functional approach that combines digital innovation, clinical best practices, and resilient supply chain strategies will position organizations to lead in the evolving bronchitis treatment arena.
Outlining Rigorous Methodological Framework and Data Collection Approaches Underpinning Comprehensive Bronchitis Treatment Insights
The research methodology underpinning these insights blends rigorous primary and secondary data collection techniques to ensure a comprehensive understanding of the bronchitis treatment landscape. Primary research involved in-depth interviews with key opinion leaders, healthcare providers, and industry executives, while advisory board consultations provided strategic perspectives on emerging therapeutic trends. Secondary research encompassed systematic reviews of peer-reviewed journals, regulatory filings, clinical trial registries, and authoritative guidelines issued by global health agencies. Data triangulation across these sources reinforced the validity of our thematic analyses and trend identifications.
Quantitative data analysis incorporated segmentation frameworks and distribution channel mapping, allowing for nuanced exploration of therapy adoption patterns across patient demographics and care settings. Qualitative insights were derived through thematic coding of expert interviews, facilitating the identification of strategic priorities and unmet clinical needs. Additionally, regional workshops and advisory panels contributed contextual intelligence on tariff impacts, regulatory nuances, and market access considerations. Throughout the process, strict data governance protocols and cross-verification procedures were applied to maintain the integrity and reliability of findings that inform strategic decision making in bronchitis treatment.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Bronchitis Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Bronchitis Treatment Market, by Drug Class
- Bronchitis Treatment Market, by Treatment Type
- Bronchitis Treatment Market, by Route Of Administration
- Bronchitis Treatment Market, by Age Group
- Bronchitis Treatment Market, by Product Form
- Bronchitis Treatment Market, by End User
- Bronchitis Treatment Market, by Distribution Channel
- Bronchitis Treatment Market, by Region
- Bronchitis Treatment Market, by Group
- Bronchitis Treatment Market, by Country
- United States Bronchitis Treatment Market
- China Bronchitis Treatment Market
- Competitive Landscape
- List of Figures [Total: 19]
- List of Tables [Total: 1908 ]
Synthesizing Strategic Perspectives and Industry Imperatives to Navigate the Evolving Bronchitis Treatment Landscape and Emerging Growth Pathways
In synthesizing the key findings, it is evident that the bronchitis treatment landscape is at an inflection point characterized by digital health integration, personalized therapy approaches, and resilient supply chain imperatives. Stakeholders must leverage segmentation insights and regional dynamics to deploy targeted interventions that address distinct patient subsets and healthcare infrastructures. A clear emphasis on antimicrobial stewardship, patient education, and innovative delivery systems will be paramount to achieving superior clinical outcomes while containing costs and curbing resistance risks.
Looking ahead, the convergence of technological advancements, strategic partnerships, and adaptive regulatory frameworks sets the stage for sustained progress in bronchitis management. Organizations that proactively embrace these trends, invest in agile manufacturing and digital platforms, and foster collaborative ecosystems will be best positioned to capture value and deliver transformative patient care. Collectively, these insights underscore a paradigm where adaptability and foresight define leadership in respiratory therapeutics.
Contact Associate Director Ketan Rohom to Acquire In-Depth Bronchitis Treatment Market Intelligence and Advance Organizational Strategies
To gain unparalleled visibility into the bronchitis treatment market and access actionable intelligence that drives strategic growth, consulting with Ketan Rohom, Associate Director of Sales & Marketing, is essential. His expertise in aligning research findings with commercial imperatives will empower your organization to refine positioning, optimize product portfolios, and accelerate time to market. Through one-on-one engagement, you will receive tailored guidance on leveraging the full breadth of this comprehensive report.
Reach out to secure your access to detailed analyses on therapy landscapes, tariff impacts, segmentation frameworks, and regional insights. Engaging directly with Ketan Rohom ensures you harness the most relevant data and expert recommendations to inform decision making and outpace competitors in the dynamic bronchitis treatment sector. Schedule a personalized briefing to explore in detail how emerging trends align with your organizational objectives and uncover opportunities for collaboration, innovation, and market expansion. This strategic conversation will equip you with clear action plans to navigate tariff dynamics, harness regional differentiators, and capitalize on evolving treatment modalities.

- How big is the Bronchitis Treatment Market?
- What is the Bronchitis Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




