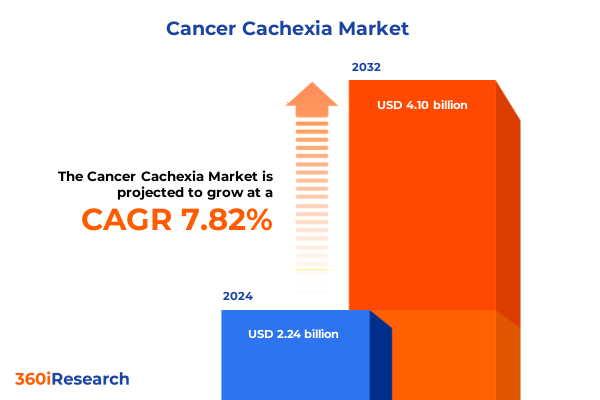
The Cancer Cachexia Market size was estimated at USD 2.37 billion in 2025 and expected to reach USD 2.50 billion in 2026, at a CAGR of 8.14% to reach USD 4.10 billion by 2032.
Exploring the multifaceted challenges of cancer cachexia, its complex pathophysiology, and the evolving therapeutic landscape in contemporary oncology care
Cancer cachexia remains a formidable challenge in oncology care, characterized by profound weight loss, muscle wasting, and metabolic dysregulation that directly impact treatment tolerance, quality of life, and overall survival. This syndrome arises from a complex interplay of inflammatory mediators, hormonal imbalances, and altered energy metabolism, creating an urgent need for targeted interventions that go beyond traditional nutritional support. Moreover, as the oncology landscape evolves with novel immuno-oncology agents and precision therapies, the importance of managing cachexia has intensified, since unaddressed wasting can undermine the efficacy and safety profiles of cutting-edge treatments.
Furthermore, the rise of patient-centric care models has highlighted the critical role of symptom management in driving adherence and outcomes, positioning cachexia therapies at the intersection of oncology and palliative disciplines. In addition, emerging evidence underscores how early identification and intervention can mitigate the irreversible sequelae of wasting, prompting multidisciplinary teams to integrate cachexia screening with standardized assessment tools. As a result, the pursuit of innovative pharmacologic, nutraceutical, and device-based modalities is accelerating, reflecting a broader commitment to holistic oncology support. Consequently, understanding the multifaceted etiology, unmet clinical needs, and evolving care pathways for cancer cachexia is indispensable for stakeholders seeking to develop, deliver, and commercialize effective solutions in this high-impact domain.
Identifying the groundbreaking scientific discoveries, regulatory advancements, and digital therapeutics that are reshaping cachexia management pathways worldwide
The landscape of cancer cachexia management is undergoing transformative shifts driven by scientific breakthroughs in mechanism-based therapies and advances in digital health. Notably, novel ghrelin receptor agonists and cytokine-targeted biologics are progressing through late-stage clinical evaluation, offering the potential to modulate appetite, anabolism, and systemic inflammation in a more precise manner than historical agents. Moreover, regulators are recognizing cachexia as a distinct clinical entity, opening pathways for accelerated approvals, adaptive trial designs, and the acceptance of patient-reported outcomes as key endpoints.
In parallel, the advent of digital therapeutics, remote nutritional monitoring, and wearable devices is reshaping care delivery by enabling real-time assessment of body composition, activity levels, and caloric intake. These innovations are facilitating proactive interventions, personalized dosing, and adherence support, thereby enhancing the overall therapeutic value proposition. Additionally, partnerships between biopharmaceutical companies and data analytics providers are harnessing artificial intelligence to develop predictive algorithms for early cachexia onset, which promises to streamline patient stratification in both clinical trials and routine practice. Taken together, these advancements are forging a new era in which cachexia is not merely managed but actively prevented and reversed, ultimately elevating patient outcomes and driving value across the healthcare continuum.
Assessing how the cumulative effects of United States tariff adjustments in 2025 shape supply chains, pricing models, and patient access to cachexia treatments
The policy environment in the United States has seen significant adjustments to import tariffs affecting pharmaceuticals, medical devices, and nutraceutical ingredients in early 2025, with cumulative duties now adding up to double-digit percentages on certain categories. These measures have introduced heightened cost pressures across the value chain, particularly for injectable administration sets, intravenous nutrition components, and specialized supplements that rely on overseas manufacturing. As a result, stakeholders are contending with longer lead times and elevated landed costs, which have in turn intensified the focus on supply security and cost containment strategies.
Consequently, many organizations are accelerating efforts to localize production, diversify supplier bases, and consolidate logistics networks to mitigate tariff exposure and ensure uninterrupted patient access. Furthermore, contract manufacturers are revising capacity plans, while distributors are renegotiating terms to offset additional fees. In the clinical setting, hospitals and home care providers are evaluating formulary adjustments and prioritizing therapeutic options that deliver the greatest clinical benefit per dollar of spending. Although these tariff changes have introduced complexity, they have also invigorated domestic manufacturing investments and fostered closer collaboration among ecosystem participants to optimize resource allocation and maintain the integrity of care delivery for patients battling cancer cachexia.
Unveiling critical segmentation insights across treatment modalities, distribution channels, administration routes, end users, and patient demographics for tailored cachexia strategies
The market’s segmentation based on treatment type reveals a diverse therapeutic ecosystem comprising medical devices, nutraceuticals and supplements, alongside a robust pharmaceutical segment. Within the pharmaceutical category, agents such as anamorelin, traditional appetite stimulants, and corticosteroids each fulfill distinct roles in counteracting anorexia and muscle catabolism. By understanding how these modalities complement or compete with each other, manufacturers and clinicians can refine treatment protocols to optimize patient-specific care pathways.
Segmentation by distribution channel highlights the critical roles of hospital pharmacies, online pharmacies, and retail pharmacies in delivering interventions to diverse patient populations. Hospital pharmacies remain pivotal for acute care settings and intravenous therapies, whereas retail and online channels are increasingly leveraged for outpatient access to oral agents and nutraceutical products. Appreciation of channel economics and patient preferences is essential for aligning logistics, pricing, and patient adherence strategies.
Exploring the route of administration underscores the dichotomy between injectable and oral therapies, with injectable formulations further subdivided into intravenous and subcutaneous options. Intravenous solutions provide rapid bioavailability and precise dosing control in critical care settings, while subcutaneous routes offer outpatient convenience. Oral formulations, including supplements and steroids, facilitate at-home management but may be subject to absorption variability.
Insights into end user categories reveal a spread across ambulatory care centers, home care environments, and hospital settings, each presenting unique operational workflows and reimbursement considerations. Hospitals drive inpatient management of severe cachexia, whereas home and ambulatory settings focus on long-term maintenance and quality-of-life preservation.
Patient demographics segmentation by age group and gender illustrates distinct prevalence and progression patterns. Adults, geriatrics, and pediatric populations experience cachexia through differing metabolic and hormonal contexts, while male and female patients may exhibit variation in symptom presentation and therapeutic response. Recognizing these demographic nuances informs targeted development, regulatory submissions, and market access approaches.
This comprehensive research report categorizes the Cancer Cachexia market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Therapy Type
- Route Of Administration
- Age Group
- Gender
- Distribution Channel
- End User
Highlighting regional dynamics influencing cachexia care with a focus on market maturity in the Americas, evolving trends in EMEA, and growth drivers in Asia-Pacific
In the Americas region, established healthcare infrastructures and high levels of oncology research investment have fostered rapid adoption of novel cachexia therapies, supported by streamlined regulatory pathways and comprehensive reimbursement frameworks. The United States leads with a well-defined guideline ecosystem and advanced home care models, while Canada and Latin American markets are gradually expanding service offerings through public-private collaborations.
Within Europe, the Middle East, and Africa, healthcare systems display a broad spectrum of maturity. Western European nations benefit from cohesive formularies and advanced clinical networks, enabling early market entry for innovative treatments. Simultaneously, emerging markets in the Middle East and Africa are leveraging philanthropic initiatives and regional manufacturing partnerships to address gaps in nutrition support and pharmacologic care, indicating a growing appetite for integrated cachexia management solutions.
The Asia-Pacific region is distinguished by its diverse demographic composition and increasing oncology incidence, driving robust demand for both established and emergent cachexia interventions. Countries such as Japan and South Korea are spearheading advanced clinical trials and digital health integrations, while markets like China and India are experiencing expansion in nutraceutical use and domestic pharmaceutical production. Cross-border collaborations and technology transfers are anticipated to accelerate equitable access and further diversify the therapeutic landscape.
This comprehensive research report examines key regions that drive the evolution of the Cancer Cachexia market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Analyzing strategic initiatives and competitive positioning of leading organizations driving innovation and partnerships in the cancer cachexia ecosystem
Leading organizations in the cancer cachexia domain are deploying strategic initiatives to reinforce their competitive positioning and accelerate innovation. Biopharmaceutical pioneers specializing in ghrelin receptor agonists have formed collaborations with academic centers to validate novel biomarkers and define patient subgroups most likely to benefit from targeted therapy. Nutraceutical and supplement manufacturers have expanded their product portfolios through acquisitions that enhance formulation stability and palatability, thereby meeting clinician and patient demands for optimized oral support.
Device manufacturers have responded to evolving clinical workflows by integrating remote monitoring capabilities into infusion pumps and wearable sensors, facilitating continuous assessment of nutritional intake and muscle mass changes. Additionally, several mid-sized biotechs are leveraging venture capital to advance anti-inflammatory cytokine inhibitors, with an eye toward licensing or acquisition by larger players seeking to diversify their oncology supportive care offerings.
Furthermore, cross-sector partnerships between pharmaceutical companies and digital health firms have produced cloud-based platforms that aggregate patient-reported outcomes, enabling health-economics analyses and payer engagement. As these collaborations deepen, a networked ecosystem is emerging in which therapeutic efficacy, patient quality of life, and economic value align to drive broader adoption of integrated cachexia management solutions.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cancer Cachexia market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AAVogen Inc.
- Actimed Therapeutics Ltd.
- Aeterna Zentaris Inc.
- Aphios Corporation
- Artelo Biosciences Inc.
- AVEO Pharmaceuticals Inc.
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- Fresenius Kabi AG
- Green Cross Wellbeing Corporation
- Helsinn Healthcare SA
- INOVIO Pharmaceuticals Inc.
- Mankind Pharma Ltd.
- Merck & Co. Inc.
- MetaFines Co., Ltd.
- NeuBase Therapeutics, Inc.
- NGM Biopharmaceuticals Inc.
- Ono Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Tetra Bio-Pharma
Providing practical and impactful strategic recommendations for industry leaders to enhance therapeutic development, supply resilience, and patient-centric outcomes in cachexia
Industry leaders should prioritize the development of patient-centric care models that integrate pharmacologic and non-pharmacologic interventions, ensuring that therapeutic regimens address both metabolic drivers and quality-of-life endpoints. By incorporating real-world evidence and patient-reported outcomes into product development and regulatory submissions, companies can demonstrate clear value propositions to payers and providers alike.
To offset ongoing tariff pressures, it is recommended to invest in regional manufacturing hubs and strengthen supply chain resilience through diversified sourcing strategies. Engaging early with contract manufacturing organizations that have proven capabilities in sterile injectable production will mitigate lead-time variability and enhance supply security.
In addition, forging alliances with digital health providers to deploy AI-enabled predictive tools can facilitate earlier detection of cachexia, enabling proactive management and optimized resource allocation. Such partnerships should encompass data-sharing agreements that comply with regulatory standards while unlocking new insights into treatment adherence and clinical outcomes.
Finally, segment-specific strategies that consider age-related metabolic differences and gender-based response patterns will refine go-to-market approaches. Tailoring formulations and dosage regimens for geriatric or pediatric populations, as well as addressing taste and administration preferences, will maximize adoption across diverse patient cohorts.
Detailing the rigorous research methodology encompassing data collection, validation processes, and analytical frameworks underpinning the cachexia market analysis
This analysis is grounded in a multifaceted research methodology that blends primary insights and secondary intelligence to ensure comprehensive coverage and validity. Primary research included structured interviews with key opinion leaders, oncology clinicians, home care specialists, and procurement managers, capturing firsthand perspectives on clinical practice, supply chain challenges, and patient needs. These dialogues were complemented by surveys administered to pharmacists and nutritionists, providing quantitative corroboration of qualitative insights.
Secondary research encompassed a systematic review of peer-reviewed journals, regulatory filings, patent databases, and conference proceedings, enabling the identification of emerging mechanisms of action and digital health innovations. Trade association reports and policy documents were analyzed to map the evolution of tariff structures and reimbursement frameworks in major markets.
All data points and thematic findings underwent rigorous validation through triangulation across sources and were subjected to expert panel review to ensure accuracy and relevance. Analytical frameworks, including PESTEL analysis for regulatory and economic factors, SWOT assessments for competitive dynamics, and segmentation modeling for patient subgroups, provided structured approaches to data synthesis. This robust methodological foundation underpins the reliability of the insights presented and supports informed decision-making in the cancer cachexia domain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cancer Cachexia market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cancer Cachexia Market, by Therapy Type
- Cancer Cachexia Market, by Route Of Administration
- Cancer Cachexia Market, by Age Group
- Cancer Cachexia Market, by Gender
- Cancer Cachexia Market, by Distribution Channel
- Cancer Cachexia Market, by End User
- Cancer Cachexia Market, by Region
- Cancer Cachexia Market, by Group
- Cancer Cachexia Market, by Country
- United States Cancer Cachexia Market
- China Cancer Cachexia Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1590 ]
Synthesizing key findings and outlining critical considerations to guide informed decision making in the evolving cancer cachexia landscape
In conclusion, the evolving landscape of cancer cachexia management underscores the convergence of scientific innovation, regulatory evolution, and supply chain adaptation in addressing a complex clinical syndrome. Breakthroughs in mechanism-based pharmacotherapies and the integration of digital health solutions are setting new benchmarks for early detection, personalized intervention, and continuous monitoring, all of which are essential for improving patient outcomes.
At the same time, policy shifts such as the 2025 tariff adjustments have highlighted the importance of supply resilience and localized manufacturing in preserving access to critical treatments. Sectioned insights into treatment modalities, distribution channels, routes of administration, end users, and demographic profiles reveal nuanced opportunities for tailored strategies that meet the distinct needs of diverse patient cohorts.
Regional analyses further demonstrate how market maturity, infrastructure development, and collaborative ecosystems vary across the Americas, EMEA, and Asia-Pacific, necessitating agile approaches to market entry and expansion. Meanwhile, competitive dynamics are being shaped by strategic partnerships, portfolio diversification, and cross-sector alliances that integrate therapeutic and digital capabilities.
Collectively, these findings illuminate a dynamic environment in which stakeholders must remain vigilant, adaptive, and collaborative. By leveraging the insights and recommendations provided, decision-makers can navigate the complexities of the cancer cachexia space and capitalize on emerging opportunities to enhance patient care and drive sustainable growth.
Contact Associate Director Sales & Marketing Ketan Rohom to access comprehensive insights and customized strategic guidance on the cancer cachexia market research report
For a deep dive into the complexities of the cancer cachexia market, tailored analysis, and strategic guidance to inform your next moves, please reach out to Ketan Rohom, Associate Director of Sales & Marketing, to secure your copy of this comprehensive market research report. Engaging with Ketan will grant you personalized insights into therapeutic trends, supply chain dynamics, and segmentation strategies needed to stay ahead in an increasingly competitive environment. By partnering directly, your organization can unlock customized data sets, gain early access to proprietary findings, and ensure that your roadmap for product development and market entry aligns with the evolving needs of patients, providers, and payers alike. Don’t miss the opportunity to leverage this authoritative resource and transform your approach to cancer cachexia treatment and care.

- How big is the Cancer Cachexia Market?
- What is the Cancer Cachexia Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




