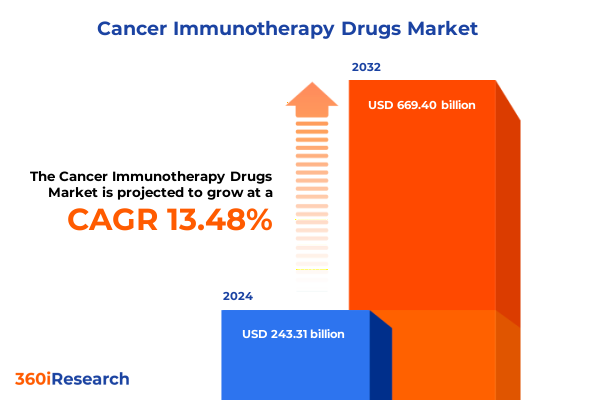
The Cancer Immunotherapy Drugs Market size was estimated at USD 271.88 billion in 2025 and expected to reach USD 294.63 billion in 2026, at a CAGR of 8.62% to reach USD 485.22 billion by 2032.
Exploring the Emergence and Unprecedented Potential of Next-Generation Cancer Immunotherapy Therapies That Are Revolutionizing Patient Outcomes
The landscape of oncology has been transformed by the advent of immunotherapy drugs, heralding a new era of precision treatment that harnesses the body’s own defense mechanisms to fight cancer. These therapies represent a significant departure from conventional cytotoxic approaches, focusing instead on modulating immune checkpoints and cellular pathways to achieve durable responses. As the field continues to evolve at an accelerated pace, stakeholders across the healthcare continuum-from researchers to clinicians to investors-must develop a clear understanding of the fundamental principles driving efficacy and safety profiles.
Moreover, the introduction of diverse modalities such as checkpoint inhibitors and cellular therapies underscores the complexity and promise inherent in modern immuno-oncology. Beyond offering new hope for patients with historically poor prognoses, these innovations are reshaping clinical guidelines and altering the standards of care. An informed perspective on the mechanisms of action and therapeutic implications sets the stage for strategic planning and resource allocation. This executive summary aims to provide decision-makers with a concise yet comprehensive orientation to the critical factors poised to influence the market trajectory of cancer immunotherapy in the years ahead.
Identifying Transformative Paradigm Shifts in the Cancer Immunotherapy Landscape Driven by Breakthrough Research and Clinical Innovation
Over the past decade, pivotal breakthroughs in molecular biology and translational research have catalyzed transformative shifts in the immunotherapy landscape. Early studies demonstrating the blockade of cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein pathways validated the concept that releasing immune checkpoints can unleash potent antitumor activity. Building on these foundational discoveries, researchers have since advanced novel constructs, including engineered CAR T cells capable of targeting tumor-associated antigens with remarkable specificity.
Concurrently, the integration of biomarker-driven patient stratification has refined patient selection and optimized response rates, while adaptive trial designs have accelerated regulatory approvals. The confluence of technological innovation, robust clinical evidence, and regulatory facilitation has fostered a fertile environment for continued pipeline expansion. Today, the immuno-oncology landscape is characterized by multifaceted combination regimens, synergistic modalities, and an emphasis on personalized medicine. These developments signal a paradigm shift away from one-size-fits-all treatments toward highly tailored interventions that promise to redefine therapeutic benchmarks.
Assessing the Cumulative Impact of New United States Trade Tariff Measures on Cancer Immunotherapy Supply Chains and Development Costs
In 2025, a new chapter unfolded as the United States implemented revised tariff measures covering key biologics, raw materials, and proprietary reagents essential for immunotherapy drug development. These levies have introduced additional cost pressures across global supply chains, affecting both established and emerging manufacturers. As a result, strategic sourcing and local manufacturing partnerships have become indispensable strategies to mitigate financial impacts and preserve margin integrity.
The ripple effects of increased import duties are particularly pronounced for smaller biotech firms reliant on specialized reagents from overseas suppliers, prompting a reassessment of clinical trial budgets and timelines. Furthermore, the need to adapt procurement strategies has spurred collaborations between pharmaceutical developers and domestic contract manufacturing organizations, aiming to reduce lead times and enhance supply chain resilience. Despite short-term challenges, these shifts are fostering a more geographically diversified production network, which may ultimately improve access and stability of advanced immunotherapy agents.
Unveiling Comprehensive Market Segmentation Insights Across Mechanisms of Action Treatment Lines and Distribution Pathways
The market’s complexity is best understood through a multi-dimensional segmentation framework that illuminates evolving trends across mechanisms of action, cancer types, treatment lines, routes of administration, end users, and distribution channels. Based on mechanism of action, therapies range from cancer vaccines and CAR T cell therapies to CTLA-4 inhibitors, cytokines, oncolytic viruses, PD-1 inhibitors, and PD-L1 inhibitors, each presenting distinct clinical attributes and development pathways. When viewed through the lens of cancer type, the field spans bladder cancer, hematologic malignancies such as leukemia, lymphoma, and multiple myeloma, kidney cancer, lung cancer subtypes including non-small cell and small cell, and melanoma, reflecting the varied immunogenicity across tumor environments.
In terms of treatment line, first-line therapies set new standards for initial intervention, while second-line approaches address resistance and relapse, and third-line and beyond therapies offer hope for heavily pretreated populations. The route of administration further differentiates products into intravenous, oral, and subcutaneous formats, influencing patient convenience, adherence, and healthcare resource utilization. End users include ambulatory care centers, hospitals, and specialty clinics, each with unique infrastructure and reimbursement considerations. Finally, distribution channels encompass hospital pharmacies, online pharmacies, and retail pharmacies, underscoring the importance of accessibility and logistical coordination. This comprehensive segmentation delivers a nuanced view of competitive dynamics and opportunity pockets across the immunotherapy landscape.
This comprehensive research report categorizes the Cancer Immunotherapy Drugs market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Therapy Type
- Route Of Administration
- Cancer Type
- Mechanism Of Action
- End User
- Distribution Channel
Analyzing Key Regional Market Dynamics Across Americas Europe Middle East Africa and Asia Pacific Therapeutic Adoption Trends
Regional dynamics play a pivotal role in shaping the adoption and commercialization of cancer immunotherapies. In the Americas, robust investment in clinical research, coupled with well-established regulatory frameworks, has accelerated the rollout of novel agents, positioning the region at the forefront of therapeutic innovation. Meanwhile, Europe, Middle East, and Africa exhibit heterogeneous market maturity, with Western European countries leading in reimbursement policies and patient access, while emerging markets are progressively building capacity through public–private partnerships and infrastructure development.
Across the Asia-Pacific region, a surge in biotech ecosystem growth and government initiatives to support local manufacturing have expanded clinical trial activity and regulatory approvals. Countries with large patient populations are actively pursuing immunotherapy adoption, leveraging real-world evidence to inform pricing negotiations and reimbursement models. Despite varied market entry barriers and regulatory complexities, these regional contours underscore global opportunities and risks, guiding strategic planning for stakeholders targeting diverse geographies.
This comprehensive research report examines key regions that drive the evolution of the Cancer Immunotherapy Drugs market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Dominant and Emerging Industry Players Driving Innovation Partnerships and Competitive Strategies in Cancer Immunotherapy
The competitive landscape of cancer immunotherapy is defined by a mix of established pharmaceutical powerhouses and agile biotechs driving next-generation modalities. Leading firms are progressively leveraging strategic alliances, licensing deals, and co-development agreements to augment their pipelines and maximize platform synergies. In parallel, emerging companies are differentiating through niche targets, novel delivery technologies, and precision immunoprofiling to address specific patient subsets.
Recent collaborations between biotech innovators and academic institutions have yielded first-in-class candidates, while partnerships with contract research organizations facilitate streamlined trial execution. Additionally, cross-sector collaborations with technology providers are enhancing digital biomarker discovery and enabling real-time monitoring of immunological responses. These intersecting strategies reflect a broader trend toward integrated ecosystems, where shared risk and collective expertise accelerate the translation of scientific breakthroughs into clinical practice.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cancer Immunotherapy Drugs market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie, Inc.
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- CARsgen Therapeutics
- Celldex Therapeutics, Inc.
- ElevateBio
- ELI Lilly and Company
- F. Hoffmann-La Roche Ltd
- Gilead Sciences, Inc.
- GlaxoSmithKline PLC
- Ikena Oncology
- Incyte Corporation
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Novartis AG
- OSE Immunotherapeutics SA
- Pfizer Inc.
- QIAGEN N.V.
- Sanofi SA
- Seattle Genetics Inc.
- Takeda Pharmaceutical Company Limited
Defining Actionable Strategic Recommendations for Industry Leaders to Capitalize on Growth Opportunities in Cancer Immunotherapy
To thrive in this competitive environment, industry leaders should prioritize a portfolio approach that balances incremental improvements in established TNF inhibitors and checkpoint blockade agents with bold investments in next-generation cellular therapies and vaccine platforms. Strengthening strategic sourcing through regional manufacturing alliances will be critical to offset tariff-induced cost pressures and ensure uninterrupted supply chains. Furthermore, integrating real-world data analytics and patient-reported outcomes into post-marketing studies can fortify evidence packages for payers and accelerate market access.
Engaging early with regulatory agencies via adaptive trial designs and rolling submissions can streamline approval timelines, while establishing centers of excellence in key markets enhances brand visibility and fosters clinician advocacy. Finally, cultivating cross-industry collaborations-spanning artificial intelligence, bioinformatics, and precision diagnostics-will unlock novel combination strategies and personalized treatment paradigms, driving sustained growth and improved patient outcomes.
Outlining Rigorous Research Methodology and Data Validation Processes Underpinning Comprehensive Cancer Immunotherapy Market Analysis
The findings presented here are underpinned by a rigorous methodology combining primary research interviews with key opinion leaders, including oncologists, pharmacologists, and regulatory experts, alongside secondary data validation from peer-reviewed journals and clinical trial registries. Quantitative analyses incorporated public and proprietary datasets to map pipeline progression, clinical trial outcomes, and regulatory milestones, ensuring a holistic perspective on development trajectories.
Complementing the quantitative component, triangulation methods were employed to corroborate insights derived from diverse sources, reducing bias and enhancing data reliability. Market mapping exercises captured competitive positioning across therapeutic classes, while scenario modeling accounted for factors such as tariff fluctuations, reimbursement policy shifts, and emerging scientific breakthroughs. This comprehensive approach guarantees that the conclusions drawn reflect the most current and credible intelligence available.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cancer Immunotherapy Drugs market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cancer Immunotherapy Drugs Market, by Therapy Type
- Cancer Immunotherapy Drugs Market, by Route Of Administration
- Cancer Immunotherapy Drugs Market, by Cancer Type
- Cancer Immunotherapy Drugs Market, by Mechanism Of Action
- Cancer Immunotherapy Drugs Market, by End User
- Cancer Immunotherapy Drugs Market, by Distribution Channel
- Cancer Immunotherapy Drugs Market, by Region
- Cancer Immunotherapy Drugs Market, by Group
- Cancer Immunotherapy Drugs Market, by Country
- United States Cancer Immunotherapy Drugs Market
- China Cancer Immunotherapy Drugs Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 3339 ]
Concluding Synthesis Highlighting Critical Insights and Emerging Opportunities in the Evolving Cancer Immunotherapy Landscape
The evolution of cancer immunotherapy has ushered in unprecedented opportunities to redefine cancer treatment paradigms and improve patient survival across a spectrum of malignancies. As therapeutic modalities diversify and regional markets mature, stakeholders must navigate shifting regulatory landscapes, supply chain complexities, and competitive pressures. The insights compiled in this executive summary underscore the importance of adopting adaptable strategies informed by segmentation analysis, regional dynamics, and tariff considerations.
Ultimately, organizations that embrace collaborative innovation, leverage real-world evidence, and proactively manage market entry barriers will be best positioned to capitalize on the transformative potential of immunotherapy. The path forward demands a synthesis of scientific excellence, strategic foresight, and operational agility to convert groundbreaking discoveries into sustainable patient benefit.
Engaging With Ketan Rohom Associate Director of Sales and Marketing to Secure the In-Depth Cancer Immunotherapy Market Research Report
To gain access to the full depth of data and strategic insights that will shape your next steps in the cancer immunotherapy arena, reach out to Ketan Rohom, Associate Director of Sales and Marketing. He will personally guide you through the comprehensive market research report, answer your questions, and ensure you have the tailored support needed to drive informed decisions. Engage with a dedicated expert who understands the nuances of immuno-oncology and can facilitate a seamless process from inquiry to acquisition. Secure your copy today to stay ahead in a rapidly evolving competitive environment and transform insight into impact.

- How big is the Cancer Immunotherapy Drugs Market?
- What is the Cancer Immunotherapy Drugs Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




