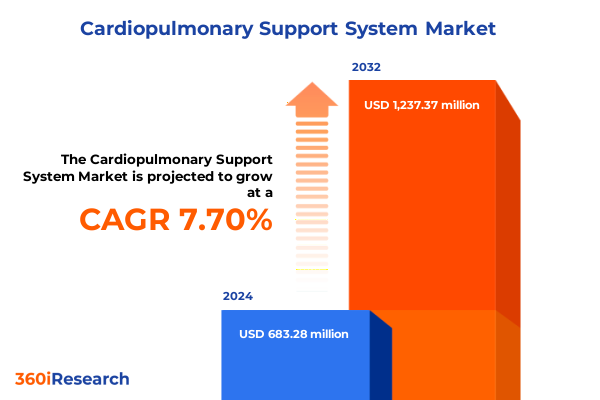
The Cardiopulmonary Support System Market size was estimated at USD 721.31 million in 2025 and expected to reach USD 782.32 million in 2026, at a CAGR of 8.01% to reach USD 1,237.37 million by 2032.
Unveiling the Critical Role of Advanced Cardiopulmonary Support Systems in Shaping Modern Healthcare Delivery and Improving Patient Survival Outcomes
The landscape of cardiopulmonary support has evolved rapidly as healthcare providers strive to enhance patient outcomes in critical care environments. Advanced support systems now play an indispensable role in stabilizing hemodynamics, enabling respiratory assistance, and providing life-saving intervention for patients experiencing cardiogenic shock, respiratory distress, or post-surgical complications. These innovations span a range of modalities, including mechanical circulatory support and extracorporeal life support systems, each designed to address specific pathophysiological challenges. As healthcare infrastructure and clinical protocols continue to advance, the integration of these technologies becomes ever more critical for improving survival rates and reducing long-term morbidity.
Moreover, the convergence of digital health with cardiopulmonary support has unlocked new possibilities for remote monitoring and predictive analytics. By embedding sensors within oxygenators, extracorporeal membrane oxygenation (ECMO) circuits, and ventilator systems, clinicians can now track patient parameters in real time, enabling proactive adjustments that mitigate complications. This shift toward data-driven therapy management underscores the strategic importance of investing in interoperable platforms that seamlessly integrate with hospital information systems. Consequently, healthcare decision-makers must prioritize solutions that not only deliver hemodynamic or respiratory stabilization but also provide actionable insights to optimize patient care pathways.
Exploring the Transformational Technological, Clinical and Economic Shifts Redefining the Cardiopulmonary Support Ecosystem Worldwide
Healthcare delivery is undergoing transformative shifts driven by technological breakthroughs, changing patient demographics, and evolving care models. The rise of minimally invasive ventricular assist devices and next-generation intra-aortic balloon pumps exemplifies the drive toward targeted hemodynamic support with reduced procedural risk. Simultaneously, the adoption of portable ECMO platforms has expanded the feasibility of extracorporeal life support beyond tertiary centers, enabling critical care transport teams to stabilize patients during interfacility transfers. As a result, mobility and accessibility have become key value drivers in system adoption.
Furthermore, regulatory bodies have streamlined approval pathways for innovative life support modalities, accelerating clinical translation. Regions with established reimbursement frameworks are incentivizing the deployment of advanced circulatory and respiratory support devices, prompting manufacturers to tailor product designs to local requirements. Additionally, the integration of artificial intelligence and machine learning algorithms into support systems has catalyzed a shift from reactive intervention to predictive management, enabling earlier detection of circuit clotting, pump failure, or patient-hostile reactions. Building on these trends, stakeholders in the ecosystem are forging strategic partnerships, leveraging cross-industry expertise to co-develop next-generation platforms that promise enhanced safety, usability, and scalability.
Assessing the Far-Reaching Consequences of 2025 United States Tariff Measures on the Cardiopulmonary Support System Supply Chain and Cost Structures
In 2025, newly implemented United States tariffs on imported medical devices and critical components have introduced profound changes to the supply chain economics of cardiopulmonary support systems. Many key inputs for ECMO circuits, oxygenator membranes, and precision-engineered pump components originate from overseas suppliers. As tariff rates increase, manufacturers face elevated input costs that must be balanced against pricing pressures from value-based care models. Consequently, procurement teams within hospitals and specialty clinics are re-evaluating supplier agreements to mitigate margin erosion.
This dynamic has spurred regional reshoring initiatives, with several OEMs investing in domestic manufacturing facilities to reduce exposure to trade fluctuations and strengthen supply continuity. In parallel, strategic inventory planning and multi-sourcing strategies have gained traction as risk mitigation measures. These adjustments are reshaping vendor relationships, shifting power dynamics toward suppliers capable of guaranteeing stable delivery schedules and cost transparency. Additionally, the tariff environment has accelerated conversations around modular system architectures, where replaceable subassemblies can be sourced from diverse geographies without requiring complete platform redesigns. Ultimately, these developments underscore the criticality of supply chain resilience as a strategic enabler for sustaining access to life-saving cardiopulmonary interventions.
Delving into Critical Market Segmentation Dimensions to Reveal Product Application Patient and End Use Dynamics Driving Growth Patterns
A nuanced examination of market segmentation reveals differentiated growth drivers across product categories, therapeutic applications, patient cohorts, and care settings. Within product portfolios, circulation support devices such as intra-aortic balloon pumps and ventricular assist devices command strategic attention for acute cardiac stabilization, while consumable elements underpin circuit longevity and safety. Concurrently, ECMO systems deliver comprehensive extracorporeal life support, complemented by oxygenators that facilitate efficient gas exchange and ventilators that address respiratory insufficiency. Together, this spectrum of offerings forms an interconnected ecosystem essential for both cardiac and respiratory critical care.
In application terms, cardiogenic shock and post cardiotomy scenarios continue to drive adoption in high-acuity units by offering targeted hemodynamic support, whereas asthma and COPD management in respiratory support applications benefits from cutting-edge ventilator technologies that adapt to patient-specific breathing patterns. Patient type also exerts a strong influence, as geriatric patients often present comorbidities requiring delicate hemodynamic balancing, while pediatric patients necessitate miniaturized circuits and tailored flow parameters. Finally, the distribution of these solutions across ambulatory surgical centers, hospitals, and specialty clinics highlights the importance of portability and ease of use, with each end use segment adopting platforms aligned to its operational and clinical demands.
This comprehensive research report categorizes the Cardiopulmonary Support System market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- Patient Type
- End Use
Uncovering Regional Variations in Adoption Infrastructure and Investment Trends Across Americas Europe Middle East Africa and Asia Pacific Markets
Regional insights illuminate how geopolitical contexts, reimbursement landscapes, and clinical infrastructure shape the deployment of cardiopulmonary support systems. In the Americas, advanced reimbursement frameworks and robust hospital networks facilitate widespread adoption of high-end ECMO platforms and ventricular assist devices. Strategic investments in tele-ICU programs further empower remote specialist oversight and expand access in underserved areas.
Across Europe, the Middle East, and Africa, heterogeneous regulatory environments drive localized product adaptations, while emerging centers of excellence in the Gulf Cooperation Council and North Africa are becoming pivotal hubs for advanced cardiac and respiratory support. Standardization efforts under pan-European health directives foster interoperability, enabling cross-border collaboration and shared best practices. Conversely, in the Asia-Pacific arena, rapid urbanization and increasing healthcare expenditures are fueling demand for scalable and cost-effective support solutions. Manufacturers are thus prioritizing regionally optimized product configurations and establishing strategic partnerships with local distributors to navigate complex regulatory frameworks and meet diverse clinical needs.
This comprehensive research report examines key regions that drive the evolution of the Cardiopulmonary Support System market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Participants and Strategic Initiatives Shaping Competitive Dynamics in the Cardiopulmonary Support System Landscape
The cardiopulmonary support ecosystem is defined by a competitive landscape where established medtech leaders and agile innovators vie for differentiation. Major global participants have expanded their footprints through targeted acquisitions, portfolio diversification, and integrated digital offerings. Strategic alliances with software providers enable real-time monitoring capabilities, while collaborations with research institutes drive the development of next-generation biomaterials and pump technologies that reduce hemolysis and improve biocompatibility.
Meanwhile, emerging players have carved niches by focusing on modular system architectures and user-centric design, addressing unmet needs in mobile extracorporeal support and outpatient respiratory therapy. These companies emphasize streamlined training modules and service-based models to lower the barrier to adoption for ambulatory centers and specialty clinics. As a result, the competitive landscape is shifting from pure device sales toward outcome-based partnerships, where vendors assume greater accountability for patient results. This evolution underscores the necessity for incumbent manufacturers to recalibrate their value propositions, integrate data-driven service solutions, and forge deeper clinical collaborations to maintain a leadership position.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cardiopulmonary Support System market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Baxter International
- BIOTRONIK SE & Co KG
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Eurosets S.r.l
- Fresenius Medical Care AG
- General Electric Company
- Getinge AB
- Jarvik Heart, Inc.
- Johnson & Johnson Services, Inc.
- Lepu Medical Technology(Beijing)Co.,Ltd.
- LivaNova PLC
- Medtronic PLC
- MicroPort Scientific Corporation
- Nipro Corporation
- Penumbra, Inc.
- Senko Medical Instrument Manufacturing Co., Ltd.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Siemens AG
- Spectrum Medical Ltd
- Stryker Corporation
- SynCardia Systems, LLC
- Terumo Corporation
Delivering Pragmatic Strategic Imperatives for Industry Stakeholders to Capitalize on Emerging Opportunities and Mitigate Risks in Cardiopulmonary Support
To capitalize on the accelerating momentum in cardiopulmonary support, industry leaders should adopt a multipronged strategy that balances innovation, operational excellence, and market engagement. First, investment in interoperable platforms that integrate pump, oxygenator, and monitoring components will foster seamless clinical workflows and unlock cross-selling opportunities. Complementing this, manufacturers should deepen partnerships with digital health firms to embed predictive maintenance and patient analytics capabilities directly into support systems.
Additionally, strengthening supply chain resilience through regional manufacturing hubs and multi-sourcing frameworks will alleviate tariff-induced cost pressures and safeguard delivery timelines. Engagement with regulatory authorities to shape favorable policy frameworks and streamline approval pathways is equally critical. Finally, providers should tailor educational initiatives and training programs to each care setting, empowering clinicians in ambulatory surgical centers, hospitals, and specialty clinics to maximize device utilization and patient outcomes. By executing this comprehensive playbook, stakeholders can secure competitive advantage and drive sustainable growth in this dynamic market.
Outlining Robust Research Framework and Rigorously Validated Data Collection Approaches Underpinning the Comprehensive Cardiopulmonary Support System Analysis
This analysis is underpinned by a rigorous, dual-phased research framework that synthesizes primary stakeholder insights and comprehensive secondary data. The primary research component encompassed in-depth interviews with leading clinicians, procurement executives, and biomedical engineers across major healthcare institutions and specialized centers. These structured discussions provided granular perspectives on device utilization patterns, clinical outcomes, and procurement decision criteria.
Moreover, extensive secondary data collection was conducted, integrating peer-reviewed journal articles, regulatory filings, patent analyses, and government health authority publications. Data triangulation techniques were applied to cross-validate insights and ensure consistency across diverse sources. Geographic representation was achieved by segmenting inputs from the Americas, Europe Middle East Africa, and Asia-Pacific regions, while methodological rigor was maintained through standardized questionnaires and expert review panels. This multifaceted approach guarantees that the findings accurately reflect real-world dynamics and support robust strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cardiopulmonary Support System market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cardiopulmonary Support System Market, by Product Type
- Cardiopulmonary Support System Market, by Application
- Cardiopulmonary Support System Market, by Patient Type
- Cardiopulmonary Support System Market, by End Use
- Cardiopulmonary Support System Market, by Region
- Cardiopulmonary Support System Market, by Group
- Cardiopulmonary Support System Market, by Country
- United States Cardiopulmonary Support System Market
- China Cardiopulmonary Support System Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1272 ]
Synthesizing Core Findings and Strategic Implications to Guide Decision Makers in Steering Sustainable Advances in Cardiopulmonary Support Solutions
In summary, the cardiopulmonary support system landscape is poised for transformative growth, driven by technological innovation, shifting care paradigms, and evolving regulatory climates. Advanced circulation support devices, ECMO platforms, oxygenators, and ventilators are increasingly integrated into holistic care pathways, supported by digital health enablers that enhance clinical decision-making. Tariff-driven supply chain challenges have catalyzed strategic shifts toward reshoring and modular architectures, underscoring the importance of resilience and adaptability. Furthermore, nuanced segmentation insights reveal differentiated adoption patterns across product types, applications, patient demographics, and care settings, while regional variations highlight the necessity for localized strategies.
As competition intensifies, leading companies must embrace outcome-based models, deepen clinical collaborations, and invest in interoperable, data-driven solutions to maintain leadership. By adhering to the actionable recommendations and leveraging this comprehensive research, decision-makers can navigate market complexities and secure sustainable advantages. Ultimately, this executive summary serves as a strategic compass for stakeholders seeking to optimize investments and drive patient-centric innovation in cardiopulmonary support.
Connect with Ketan Rohom for Direct Access to Unparalleled Cardiopulmonary Support System Market Intelligence and Strategic Guidance
To secure the full breadth of insights and actionable intelligence encapsulated in this comprehensive market research report, we invite you to connect directly with Ketan Rohom, Associate Director of Sales & Marketing. Engage with Ketan to explore how the detailed analyses, strategic frameworks, and market segmentation revelations can directly inform your investment decisions, technology roadmaps, and partnership strategies. By reaching out to Ketan, you gain tailored guidance on navigating complex regulatory landscapes, optimizing product portfolios across circulation support devices, ECMO systems, and ventilators, and leveraging regional growth vectors in the Americas, Europe Middle East Africa, and Asia-Pacific.
With Ketan’s deep expertise in cardiopulmonary support systems, you can access personalized consultations that align with your unique organizational objectives. Whether you seek to refine go-to-market strategies, evaluate supply chain resilience in light of recent tariff shifts, or identify high-potential end markets within ambulatory surgical centers and specialty clinics, this direct engagement will enable you to transform data into strategic advantage. Elevate your market positioning and accelerate innovation by partnering with Ketan Rohom today to purchase the definitive report and secure a competitive edge in an evolving healthcare ecosystem.

- How big is the Cardiopulmonary Support System Market?
- What is the Cardiopulmonary Support System Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




