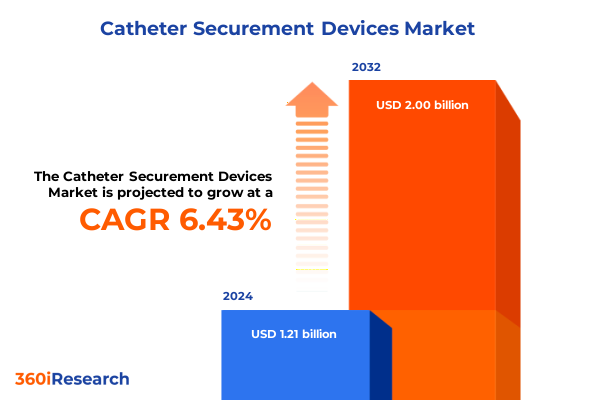
The Catheter Securement Devices Market size was estimated at USD 1.27 billion in 2025 and expected to reach USD 1.34 billion in 2026, at a CAGR of 6.66% to reach USD 2.00 billion by 2032.
Pioneering Patient Safety Through Securement: Understanding the Critical Role of Catheter Stabilization Technologies in Modern Healthcare
Intravenous and arterial catheterization represent foundational procedures in contemporary healthcare delivery, underpinning therapies from continuous medication infusions to hemodynamic monitoring. Despite their clinical indispensability, catheters introduce risks including microbial colonization, percutaneous migration, and inadvertent dislodgement, all of which can precipitate bloodstream infections, vascular trauma, and device failure. In response, securement technologies have become essential in stabilizing catheter hubs, minimizing mechanical stress at insertion sites, and reducing the incidence of avoidable complications. Continued innovation in this realm is critical as clinicians and patients alike seek enhanced safety and efficiency in invasive therapies.
Securement devices encompass a diverse range of solutions, including adhesive patches, integrated dressings, subcutaneous anchors, and suture-based systems. Each design caters to specific clinical scenarios, from short-term peripheral intravenous catheters in acute settings to long-term tunneled central venous lines in home infusion. By integrating securement into broader infection control strategies, healthcare providers can uphold aseptic techniques and adhere to evidence-based recommendations. The Centers for Disease Control and Prevention underscores the importance of using sutureless securement devices to mitigate infection risk around intravascular catheter insertion sites.
Emerging Innovations and Regulatory Advances Are Redefining Catheter Securement Solutions and Patient Outcomes Across Clinical Settings
Recent years have witnessed a wave of transformative advances in catheter securement, with manufacturers embedding protective and functional enhancements directly into device architecture. The convergence of stabilization and dressing technologies enables integrated systems that provide robust adhesion, microbial barriers, and site visibility in a single application. For instance, combined PICC/CVC securement systems leverage medical-grade silicone adhesives alongside transparent, chlorhexidine-impregnated films to deliver extended wear time, gentle removal, and continuous antimicrobial activity without sacrificing insertion-site monitoring. Concurrently, novel catheter adhesives are emerging that combine securement, hemostatic sealing, and antimicrobial properties, effectively uniting multiple care functions within a single product design.
Regulatory oversight has evolved in tandem with technological progress to ensure safety and performance. The U.S. Food and Drug Administration recently reclassified the intravenous catheter force-activated separation device under Class II special controls, mandating requirements for separation force, leak resistance, and microbial ingress testing to uphold sterility and functionality. Subcutaneous anchoring catheters are likewise governed by detailed 510(k) frameworks that demand biocompatibility and robust anchorage below the skin surface to optimize long-term stability.
International consensus standards have also been updated to reflect best practices in sharps protection and device integrity. The second edition of ISO 23908, published in late 2024, establishes stringent testing protocols for sharps injury prevention in catheter introducers and securement devices, reinforcing user safety and advancing global harmonization in device standards.
Assessing the Broad Economic and Operational Implications of 2025 United States Trade Tariffs for Medical Device Suppliers and Healthcare Providers
In response to ongoing trade policy shifts, the United States implemented an expanded set of Section 301 tariffs that took effect on January 1, 2025, raising duties on a spectrum of medical goods. Notably, tariffs on surgical and non-surgical respirators, face masks, rubber medical gloves, and syringe and needle assemblies increased substantially, with some categories subject to duties of up to 100% on direct imports from China. Although catheter securement devices are not explicitly listed, the broader escalation of medical device tariffs has ripple effects across global supply chains, elevating component costs and logistics expenses for device manufacturers and end users.
Parallel negotiations between the U.S. and the European Union signal further complexity. Reports indicate that absent a finalized trade deal by August 1, tariffs of up to 30% on European imports-including select medical devices-could be imposed, even as both sides prepare to waive duties on certain critical goods if an agreement is reached. This tariff volatility compels manufacturers to recalibrate sourcing strategies, explore alternate manufacturing locales, and advocate for exemptions on essential healthcare supplies to avoid inflationary pressures that could impair patient care budgets.
The compounded effect of U.S.-China Section 301 duties and potential U.S.-EU measures underscores the urgency for industry stakeholders to engage with policymakers, secure temporary exclusions, and investigate near-shoring opportunities. By proactively managing tariff exposures, medical device suppliers can safeguard margin integrity and ensure uninterrupted access to life-saving securement solutions in hospital, ambulatory, and home-care environments.
Deep Dive Into Product, Catheter, Application and End-User Segments Reveals Nuanced Drivers of Securement Device Adoption and Growth Dynamics
Analyzing the market through the lens of product type reveals that adhesive securement devices form the cornerstone of short-term peripheral vascular care, favored for their ease of application and comfortable wear in acute settings. Overlaid on this foundation, integrated securement and dressing products are gaining adoption in intensive care units, where the convergence of stabilization and infection prevention is critical. Subcutaneous anchoring devices, historically reserved for tunneled central venous catheters, are now extending into long-term infusion therapies, driven by patient mobility and reduced need for periodic re-suturing. Meanwhile, suture securement remains relevant in surgical and emergent applications where mechanical fixation offers rapid deployment under variable field conditions.
In terms of catheter type, central venous catheters continue to underpin the majority of securement innovation, as their use in chemotherapy, parenteral nutrition, and hemodynamic monitoring demands both robust fixation and infection risk mitigation. Dialysis catheters benefit from specialized dressings and anchors that accommodate frequent access, while arterial catheter stabilization has trended toward low-profile adhesive anchors that support continuous blood pressure monitoring without hampering patient mobility. Peripheral intravenous catheters benefit from streamlined, user-friendly securement patches that simplify nursing workflows and reduce dressing changes.
Segmenting by clinical application, acute catheters in hospital settings maintain the highest volume of securement device utilization, reflecting the concentration of short-term vascular access in emergency and perioperative units. At the same time, chronic catheters deployed in home healthcare and outpatient infusion centers underscore a growing need for devices engineered for multi-day wear and patient self-management. Hemodialysis catheters occupy a specialized niche, where durability and infection control converge under demanding reuse protocols.
From the perspective of end users, hospitals anchor the market with comprehensive procurement frameworks that prioritize FDA-cleared systems and institutional protocols. Ambulatory surgical centers and specialty clinics are accelerating adoption of integrated securement solutions to streamline patient turnover and minimize complication rates. Meanwhile, home healthcare providers are emerging as influential buyers, driving demand for adhesives and anchoring systems designed for non-clinical settings and patient-centered ease of use.
This comprehensive research report categorizes the Catheter Securement Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Catheter Type
- Application
- End User
Regional Healthcare Infrastructure and Policy Landscapes Shape Diverse Growth Trajectories for Catheter Securement Devices Across Global Markets
Across the Americas, the United States leads securement device utilization, buoyed by sophisticated hospital networks, robust reimbursement pathways for vascular access interventions, and an accelerating shift toward outpatient infusion services. Latin American markets exhibit nascent but growing appetite for cost-effective adhesive anchors and integrated dressings as health ministries invest in infection control programs to curb catheter-associated complications.
In Europe, Middle East & Africa, regulatory harmonization through the EU Medical Device Regulation has elevated standards for product safety and clinical performance, prompting manufacturers to localize production and reinforce compliance capabilities. Demand in the Middle East is underpinned by investments in tertiary care infrastructure, while African markets are characterized by selective adoption of low-cost securement solutions that align with constrained healthcare budgets and supply chain limitations.
Asia-Pacific demonstrates dynamic growth as governments expand healthcare coverage and upgrade critical care units. China’s domestic industry is ramping up production of adhesive and sutureless devices to serve rapidly expanding urban hospital systems, while Japan and South Korea emphasize advanced integrated dressings that support multi-day wear and antimicrobial efficacy. Southeast Asian markets reflect a dual demand for both premium and value-oriented securement options, driven by diverse economic conditions and varied levels of clinical sophistication.
This comprehensive research report examines key regions that drive the evolution of the Catheter Securement Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Competitive Strategies and Portfolio Innovations Among Leading Manufacturers Are Shaping the Evolution of Catheter Securement Solutions
Leading manufacturers are redefining the securement landscape through portfolio diversification, strategic partnerships, and targeted R&D investments. 3M, for example, has integrated its PICC/CVC stabilization devices with Tegaderm I.V. Advanced Securement Dressings to deliver a combined solution that offers site visibility, antimicrobial protection, and repositionable silicone adhesion in a single system. BD’s StatLock line leverages precision-engineered anchor pads and hypoallergenic adhesives to secure a wide spectrum of peripheral and arterial catheters, emphasizing user training and skin prep protocols to optimize device performance and reduce premature uplift.
Smaller innovators such as H.B. Fuller’s Adhezion Biosystems have introduced multifunctional catheter adhesives that unify securement, hemostasis, and antimicrobial sealing, addressing the evolving needs of co-located dressing and fixation practices. Teleflex continues to expand its StatLock anchoring family through custom stabilization kits that combine nerve-stimulating catheter connectors with integrated securement and draping components for regional anesthesia applications.
Across the board, industry leaders are pursuing ecosystem approaches that bundle securement devices with dressings, skin prep, and educational resources to reinforce best practices. These strategies enhance clinical value, streamline supply chain complexity, and foster long-term relationships with healthcare systems seeking turnkey vascular access solutions.
This comprehensive research report delivers an in-depth overview of the principal market players in the Catheter Securement Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- 3M Company
- Avanos Medical, Inc.
- Baxter International Inc.
- Becton, Dickinson and Company
- BioDerm, Inc.
- Centurion Medical Products Corporation
- Dale Medical Products, Inc.
- DeRoyal Industries, Inc.
- ICU Medical, Inc.
- Medtronic plc
- Owens & Minor, Inc.
- Smith & Nephew plc
- Teleflex Incorporated
- Terumo Corporation
- TIDI Products, LLC
- Vygon SAS
Strategic Roadmap for Stakeholders to Capitalize on Technological, Regulatory and Market Trends in Catheter Securement Device Development
To thrive in an environment defined by technological disruption and regulatory evolution, industry participants must pursue a multifaceted strategy. Investment in truly integrated securement and dressing platforms can unlock incremental value by simplifying clinical workflows and improving patient comfort. Parallel development of antimicrobial adjuncts and bioactive adhesives will reinforce infection prevention objectives and align with hospital quality metrics.
Given the unpredictability of trade policies, manufacturers should cultivate diversified supply chains, evaluate near-shoring opportunities for critical components, and proactively engage with regulators to secure exemptions for essential device categories. Collaborative partnerships with home healthcare providers and ambulatory centers will facilitate market entry into fast-growing outpatient segments, while clinician education initiatives will be essential to drive adherence to best practices and capitalize on advanced securement features.
Embracing digital monitoring-such as sensor-enabled anchors that detect excess tension or moisture ingress-offers a roadmap for next-generation securement that extends beyond mechanical stabilization to active site surveillance. Aligning these innovations with sustainable materials and circular-economy design principles can further differentiate offerings and meet emerging environmental stewardship targets.
Rigorous Multi-Source Research Framework Combining Expert Interviews, Regulatory Analysis and Patent Review to Illuminate Securement Device Market Dynamics
The findings herein are grounded in a rigorous, multi-source research framework. Primary interviews were conducted with vascular access nurses, procurement leaders, and device engineers to capture firsthand insights into clinical preferences and operational challenges. Secondary research encompassed a comprehensive review of regulatory filings, Federal Register notices, and international consensus standards, ensuring alignment with the latest 510(k) classifications, ISO protocols, and tariff schedules.
To corroborate emerging trends, patent landscaping and product datasheet analyses were performed to identify key innovation pathways and proprietary adhesive formulations. Trade and customs data informed assessments of supply chain vulnerabilities linked to Section 301 tariffs and U.S.-EU trade negotiations. Finally, iterative validation workshops with subject matter experts enabled refinement of strategic recommendations and ensured the relevance of segmentation and regional analyses.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Catheter Securement Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Catheter Securement Devices Market, by Product Type
- Catheter Securement Devices Market, by Catheter Type
- Catheter Securement Devices Market, by Application
- Catheter Securement Devices Market, by End User
- Catheter Securement Devices Market, by Region
- Catheter Securement Devices Market, by Group
- Catheter Securement Devices Market, by Country
- United States Catheter Securement Devices Market
- China Catheter Securement Devices Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 954 ]
Synthesizing Insights on Clinical Impact, Market Shifts and Strategic Imperatives to Navigate the Future of Catheter Securement Technologies with Confidence
As healthcare systems continue to adapt to cost pressures, patient safety imperatives, and shifting care modalities, securement devices stand at the intersection of clinical efficacy and operational efficiency. Technological advances-from multifunctional adhesives and integrated dressings to sensor-enabled stabilization platforms-are driving a new era of patient-centric vascular access.
Simultaneously, evolving regulatory classifications and trade policy uncertainties underscore the need for agile supply chain management and proactive stakeholder engagement. By dissecting market segments, regional nuances, and competitive landscapes, this executive summary offers a strategic compass for manufacturers, providers, and policymakers alike. The future of catheter securement lies in harmonizing device innovation with evidence-based infection control, streamlined clinician workflows, and resilient global sourcing, ensuring that patients everywhere benefit from safer, more reliable access solutions.
Unlock Comprehensive Catheter Securement Market Intelligence by Connecting with Associate Director Ketan Rohom for Tailored Research and Purchasing Options
To explore a detailed analysis, robust data sets, and strategic insights tailored to your organization’s needs, reach out to Ketan Rohom, Associate Director of Sales & Marketing for an in-depth discussion on acquiring the complete catheter securement devices market research report.

- How big is the Catheter Securement Devices Market?
- What is the Catheter Securement Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




