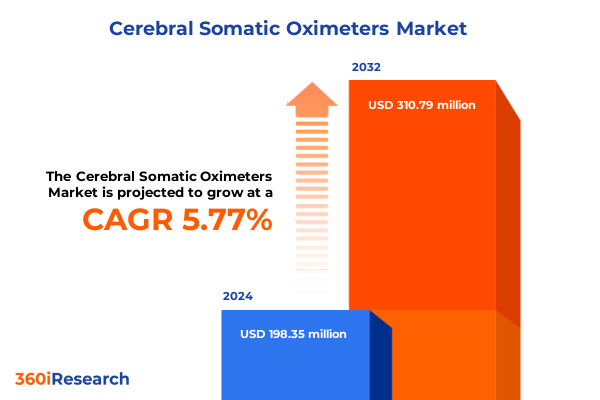
The Cerebral Somatic Oximeters Market size was estimated at USD 206.83 million in 2025 and expected to reach USD 221.30 million in 2026, at a CAGR of 5.98% to reach USD 310.78 million by 2032.
Unveiling the Critical Role of Cerebral Somatic Oximeters in Modern Healthcare to Enhance Patient Safety, Clinical Decision Making, and Operational Efficiency
Cerebral somatic oximeters have emerged as indispensable tools for clinicians striving to maintain optimal cerebral oxygenation during high-risk medical procedures and critical care scenarios. These advanced monitoring devices employ near-infrared spectroscopy and time-domain analysis to provide real-time, noninvasive measurements of regional tissue oxygen saturation. As healthcare providers confront an escalating demand for improved patient safety and streamlined perioperative workflows, cerebral somatic oximetry is positioned at the forefront of innovation. The integration of portable and fixed systems enables seamless adoption across diverse clinical environments, from high-acuity operating rooms to neonatal intensive care units. Moreover, recent technological refinements have enhanced signal processing algorithms, delivering greater accuracy, reduced noise interference, and intuitive user interfaces that facilitate rapid clinical decision making.
In tandem with technological advancements, the broader healthcare ecosystem is placing renewed emphasis on value-based care, driving adoption of tools that demonstrably reduce complications, shorten recovery times, and lower overall treatment costs. By continually monitoring cerebral oxygenation, medical teams can proactively identify hypoxic events, mitigate the risk of neurological injury, and tailor therapeutic interventions in real time. As regulatory authorities around the world strengthen guidelines for patient monitoring standards, the demand for reliable, evidence-based oximetry solutions continues to accelerate. This confluence of clinical need, technological capability, and policy momentum underscores the critical role of cerebral somatic oximeters in shaping future care paradigms.
Identifying Revolutionary Shifts Reshaping Cerebral Somatic Oximetry through Technological Innovation, Data Analytics, and Adapting Clinical Protocols
The landscape of cerebral somatic oximetry is undergoing transformative shifts driven by rapid technological innovation, expanded clinical applications, and the incorporation of advanced data analytics. Next-generation devices now integrate artificial intelligence and machine learning algorithms that analyze oxygenation trends, predict potential desaturation events, and provide decision support through anomaly detection. These capabilities are poised to enhance clinical confidence, guiding interventions before irreversible damage occurs. Concurrently, the miniaturization of sensor modules and the advent of wireless connectivity have enabled continuous monitoring outside traditional perioperative settings, opening new frontiers in home care and ambulatory surgical centers.
Clinical protocols are also evolving to embrace proactive neuromonitoring strategies. In cardiac surgery, for instance, the adoption of cerebral oximetry is expanding beyond adult procedures to pediatric and neonatal populations, where precision monitoring of full-term and preterm neonates can significantly reduce the incidence of neurodevelopmental impairment. Meanwhile, in intensive care units, continuous tracking of adult and pediatric patients supports early identification of ischemic events in stroke monitoring and traumatic injury management. This broader clinical utilization is reinforced by practice guidelines from leading societies, emphasizing the incorporation of regional oxygen saturation monitoring into multimodal neuromonitoring protocols.
Analyzing the Multifaceted Impact of 2025 United States Tariffs on Cerebral Somatic Oximeter Supply Chains and Market Dynamics
The introduction of new tariff measures by the United States government in early 2025 has imparted multifaceted effects on the cerebral somatic oximeter industry. Increased duties on imported optical components, including sensor materials and laser diodes, have elevated upstream manufacturing costs. As a result, device makers have faced pressure to reassess supply chain strategies, with several electing to source critical components domestically or diversify regional procurement to mitigate cost volatility. This shift has also accelerated collaborations between original equipment manufacturers and specialized suppliers in North America, aiming to develop vertically integrated production models that enhance cost control and reduce lead times.
Despite these challenges, higher input costs have spurred innovation in materials science and manufacturing processes. Leading players have invested in research initiatives to identify alternative polymer substrates for sensor casings and to optimize printed circuit board assembly techniques that conserve materials without compromising performance. These strategies have gradually offset tariff-induced inflation, enabling manufacturers to preserve competitive pricing structures. Meanwhile, the redistribution of manufacturing footprints has contributed to more resilient regional ecosystems, supporting local job creation and reinforcing strategic supply chain redundancies in the face of geopolitical uncertainties.
Exploring In-Depth Segmentation Insights Across Applications, End Users, Technology Types, Products, and Sales Channels Driving Market Precision
A nuanced understanding of market segmentation reveals distinct dynamics across clinical application areas, end-user environments, technology modalities, product configurations, and distribution channels. In the domain of cardiac surgery, adult procedures have traditionally driven demand, yet pediatric cardiac operations are experiencing rapid uptake due to growing awareness of long-term neurodevelopmental implications. The intensive care unit segment similarly bifurcates into adult and pediatric settings, each with unique monitoring protocols and staffing models. Meanwhile, neonatal monitoring for both full-term and preterm populations remains a critical growth frontier, given the heightened vulnerability of neonates to hypoxic events. Neurosurgical applications in stroke monitoring, traumatic injury management, and tumor resection are also expanding, supported by evidence demonstrating improved surgical outcomes through continuous tissue oxygenation tracking.
End-user segmentation spans hospitals, ambulatory surgical centers, and home care settings. Within hospitals, government-owned institutions continue to upgrade their neuromonitoring capabilities, while private hospitals focus on premium service differentiation. Ambulatory surgical centers, ranging from freestanding facilities to hospital-affiliated units, are integrating portable systems to expedite patient throughput and reduce hospitalization durations. In home care, remote oximetry platforms extend critical care monitoring into post-discharge recovery phases. Technological choices further stratify the market, with near-infrared spectroscopy maintaining prominence for noninvasive continuous monitoring, frequency domain systems offering enhanced spatial resolution, and time-domain devices delivering deeper tissue penetration. Additionally, fixed installations in dedicated monitoring suites compete alongside portable units designed for mobility. Distribution has evolved from traditional offline sales networks toward a growing online presence, with manufacturers’ websites and third-party e-retailers making devices more accessible to a broader range of end users.
This comprehensive research report categorizes the Cerebral Somatic Oximeters market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Technology Type
- Application
- End User
- Channel
Uncovering Regional Dynamics Across Americas, EMEA, and Asia-Pacific Highlighting Growth Drivers, Regulatory Environments, and Adoption Trends
Geographic dynamics in the cerebral somatic oximeter landscape illustrate diverse drivers and regulatory frameworks across the Americas, Europe, Middle East and Africa, and Asia-Pacific. In North and South America, government health agencies have incorporated regional oxygen saturation monitoring into clinical guidelines for cardiac and neurosurgical procedures, fueling demand in both public and private care settings. Latin American nations are also emerging as manufacturing hubs, benefiting from favorable trade agreements and a growing base of biomedical engineering expertise.
In Europe, Middle East and Africa, stringent regulatory approvals and reimbursement policies dictate adoption patterns. Western European countries, led by Germany and the United Kingdom, prioritize the integration of neuromonitoring technologies in complex surgical interventions, backed by robust healthcare funding. In contrast, emerging markets in the Middle East and Africa are characterized by selective adoption in tertiary care centers, often driven by medical tourism initiatives. Regulatory harmonization efforts by the European Medicines Agency have streamlined device approvals, enhancing market access for new entrants.
Across Asia-Pacific, rapid modernization of healthcare infrastructure in countries such as China, India, and Australia is driving widespread adoption. Government-led funding programs and public-private partnerships in these nations have accelerated procurement of advanced monitoring systems, while regional manufacturers are scaling production capacity to serve both domestic and export markets. This convergence of policy support, cost-effective manufacturing, and rising clinical awareness has positioned the Asia-Pacific region as a pivotal growth engine for cerebral somatic oximetry.
This comprehensive research report examines key regions that drive the evolution of the Cerebral Somatic Oximeters market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Strategic Moves of Leading Cerebral Somatic Oximeter Providers with Partnerships, Innovations, and Competitive Differentiation
The competitive landscape for cerebral somatic oximeters is defined by a blend of established medical device conglomerates and agile specialized innovators. Leading electronics and healthcare companies have fortified their market positions through strategic partnerships, mergers, and acquisitions that broaden their product portfolios and enhance R&D capabilities. For instance, collaborations between sensor technology pioneers and clinical monitoring solution providers have yielded integrated platforms combining oximetry with multimodal neuromonitoring.
Innovation leadership is further evidenced by the launch of next-generation devices featuring augmented reality displays, integrated alarm management, and cloud-based analytics dashboards. These enhancements enable care teams to visualize oxygenation trends alongside hemodynamic data in real time, fostering interdisciplinary coordination. Moreover, several incumbents are leveraging digital health platforms to offer remote monitoring services, expanding their footprint into telehealth and post-acute care segments. Emerging companies, meanwhile, are concentrating on niche applications such as neonatal and portable solutions, investing in lean manufacturing techniques and localized distribution networks to achieve competitive differentiation.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cerebral Somatic Oximeters market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- CAS Medical Systems, Inc.
- Edwards Lifesciences Corporation
- GE Healthcare Technologies Inc.
- Hamamatsu Photonics K.K.
- HyperMed Imaging, Inc.
- ISS, Inc.
- Koninklijke Philips N.V.
- Masimo Corporation
- Medtronic plc
- Mespere LifeSciences Inc.
- Nihon Kohden Corporation
- Nonin Medical, Inc.
- Opto Circuits (India) Ltd.
- Ornim Medical Ltd.
- Terumo Cardiovascular Systems Corporation
Offering Actionable Strategies for Industry Leaders to Capitalize on Emerging Opportunities and Mitigate Potential Market Risks
Industry leaders seeking to capitalize on the evolving cerebral oximetry landscape should adopt a multipronged strategy that emphasizes technological agility, market expansion, and stakeholder engagement. First, prioritizing modular product architectures will enable rapid customization for specific clinical segments, from pediatric cardiac surgery suites to home-based post-discharge monitoring kits. By offering scalable platforms, manufacturers can address diverse end-user needs with minimal redevelopment costs.
Second, forging alliances with healthcare providers and academic research centers will accelerate clinical validation of advanced features such as predictive analytics and machine learning–driven alerts. Joint clinical studies and real-world evidence initiatives will enhance product credibility, supporting favorable reimbursement scenarios. Third, expanding global manufacturing footprints through regional partnerships will mitigate tariff-related cost pressures and ensure supply continuity. Lastly, investing in digital ecosystem development-encompassing cloud-based data management, remote device management, and virtual training modules-will foster deeper customer engagement and create recurring revenue streams beyond device sales.
Detailing a Rigorous Research Methodology Combining Primary Interviews, Secondary Analysis, and Data Triangulation to Ensure Robust Insights
This analysis is underpinned by a rigorous research methodology that integrates primary interviews, secondary data analysis, and comprehensive data triangulation techniques. Primary insights were obtained through one-on-one discussions with clinicians, biomedical engineers, and procurement specialists across key regions, providing firsthand perspectives on technology adoption drivers, clinical outcomes, and purchasing considerations. Secondary research encompassed a thorough review of industry publications, regulatory filings, clinical trial registries, and patent databases to map the competitive landscape and technological evolution.
Quantitative data was extracted from regional health ministry reports, hospital procurement records, and trade association statistics to validate market trends and supply chain dynamics. Qualitative assessment was conducted by subject matter experts who synthesized insights around clinical protocols, reimbursement policies, and material innovations. To ensure robustness, all findings were cross-verified using data triangulation, reconciling discrepancies between independent sources. The research framework adhered to international best practices for market intelligence, ensuring transparency, reproducibility, and strategic relevance.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cerebral Somatic Oximeters market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cerebral Somatic Oximeters Market, by Product Type
- Cerebral Somatic Oximeters Market, by Technology Type
- Cerebral Somatic Oximeters Market, by Application
- Cerebral Somatic Oximeters Market, by End User
- Cerebral Somatic Oximeters Market, by Channel
- Cerebral Somatic Oximeters Market, by Region
- Cerebral Somatic Oximeters Market, by Group
- Cerebral Somatic Oximeters Market, by Country
- United States Cerebral Somatic Oximeters Market
- China Cerebral Somatic Oximeters Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 2067 ]
Concluding the Executive Summary with Key Takeaways Emphasizing Market Momentum, Strategic Imperatives, and Future Outlook
The cerebral somatic oximeter market is poised for sustained advancement as stakeholders align around patient-centric care models, technological innovation, and resilient supply chain strategies. Critical success factors include the integration of advanced analytics, expansion into emerging clinical segments such as neonatal and home-based monitoring, and the establishment of strategic regional partnerships to offset geopolitical risks. Companies that proactively invest in modular, software-enabled platforms and foster collaborative clinical research will be best positioned to capture value in this dynamic landscape.
Moreover, the convergence of healthcare digitization and value-based reimbursement frameworks underscores the need for continuous evidence generation and outcome-based pricing models. As the market matures, differentiation through comprehensive service offerings and data-driven insights will become increasingly important. By adhering to the strategic imperatives highlighted herein, industry participants can navigate evolving regulatory environments, optimize operational efficiencies, and secure lasting competitive advantage in the cerebral somatic oximetry space.
Engaging Decision Makers with a Clear Call to Action to Connect with Ketan Rohom and Secure Exclusive Market Intelligence
To explore these critical insights further and gain a competitive edge, we invite you to connect directly with Ketan Rohom, Associate Director, Sales & Marketing. By securing this comprehensive report, you will gain unparalleled visibility into evolving market dynamics, regulatory developments, and technological breakthroughs shaping cerebral somatic oximetry. Our tailored briefing session with Ketan will enable you to align strategic initiatives and investment priorities with the most current intelligence. Reach out to Ketan Rohom through our sales portal to arrange a personalized consultation and access the full breadth of data-driven recommendations. Empower your organization with the knowledge needed to capitalize on emerging growth areas, optimize operational efficiencies, and mitigate evolving market risks. Secure your copy today to stay ahead of the curve and drive sustained success in the cerebral somatic oximeter landscape.

- How big is the Cerebral Somatic Oximeters Market?
- What is the Cerebral Somatic Oximeters Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




