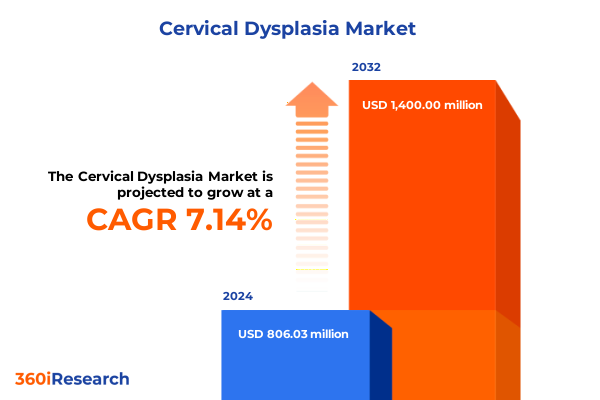
The Cervical Dysplasia Market size was estimated at USD 854.88 million in 2025 and expected to reach USD 907.90 million in 2026, at a CAGR of 7.30% to reach USD 1,400.22 million by 2032.
Comprehensive overview of cervical dysplasia market dynamics highlighting the critical need for innovation in diagnostic, screening, and treatment pathways
The landscape of cervical dysplasia is marked by a convergence of technological innovation and growing public health initiatives, triggering a pivotal moment for stakeholders across the healthcare continuum. Recent advances in molecular diagnostics, coupled with heightened awareness of human papillomavirus (HPV) transmission dynamics, have accelerated demand for more precise detection methods. Simultaneously, evolving clinical guidelines are driving adoption of both established cytology and emerging point-of-care HPV testing, fostering a diversified ecosystem of screening approaches. Within treatment settings, the refinement of minimally invasive options such as laser ablation and electrosurgical excision has enhanced patient outcomes and procedural efficiency, while parallel progress in cryotherapy device ergonomics underscores the industry’s commitment to accessibility.
Against this backdrop, providers and manufacturers are challenged to collaborate across diagnostic laboratories, ambulatory surgical centers, and specialty clinics to integrate seamless workflows that balance cost pressures with a patient-centric ethos. As the market pivots towards data-driven decision making, real-world evidence is becoming indispensable for validating clinical utility and securing favorable reimbursement pathways. Transitioning from traditional paradigms to an integrated model that unites biopsy, colposcopy, visual inspection, and advanced analytics is essential for stakeholders aiming to fortify their positioning in a rapidly transforming market.
Breakthrough innovations in diagnostics and therapeutics are reshaping the cervical dysplasia landscape to advance accessibility and clinical precision
The cervical dysplasia market is experiencing transformative shifts as emerging technologies redefine diagnostic and therapeutic standards. Artificial intelligence-enhanced cytology platforms are streamlining slide interpretation, reducing false negatives and accelerating clinical decision timelines. At the same time, the integration of multiplex HPV testing into routine screening algorithms is expanding detection capabilities to cover a broader spectrum of high-risk viral genotypes. These advancements are complemented by evolving telehealth ecosystems that enable remote colposcopic assessment, facilitating timely referrals and resource allocation in underserved regions.
In parallel, the treatment landscape is pivoting towards device modularity and portability. Next-generation cryotherapy units are designed for low-resource environments, while portable electrosurgical tools and compact laser systems support same-day interventions. This shift underscores a broader trend towards democratizing access to care, aligning with global health directives that prioritize early intervention. As stakeholders navigate these transitions, strategic partnerships among diagnostic laboratories-both hospital based and independent-and technology vendors will be critical for fostering scalable solutions that meet the dual demands of clinical efficacy and operational efficiency.
Analysis of how newly established United States medical device tariffs in 2025 have reshaped procurement dynamics and cost structures across the cervical dysplasia ecosystem
In 2025, newly implemented United States tariffs on select medical devices and components have exerted a cumulative impact on the cervical dysplasia market, prompting a recalibration of procurement strategies across the supply chain. Manufacturers reliant on imported components for biopsy forceps and colposcopes have encountered increased input costs, driving a ripple effect that has been felt by hospitals and ambulatory surgical centers as they reconcile budget constraints with quality imperatives. The tariffs have also influenced pricing negotiations for electrosurgical units and laser systems, compelling buyers to explore alternative sourcing channels and local assembly partnerships.
Despite these headwinds, several domestic device producers have leveraged tariff-induced market shifts to expand manufacturing capabilities stateside, mitigating exposure to import duties. Concurrently, diagnostic laboratories are optimizing operational efficiencies by consolidating procurement of cytology and HPV testing reagents from suppliers with integrated supply-chain models that absorb tariff fluctuations. While short-term cost pressures have emerged, the market is demonstrating resilience through strategic realignment of vendor relationships and a revitalized emphasis on regional production networks.
In-depth segmentation insights reveal how diagnostic, screening, and therapeutic categories intersect with device portfolios and end-user workflows to drive market priorities
Segmentation analysis uncovers nuanced insights into application-driven priorities within the cervical dysplasia market, revealing that screening modalities anchor early detection efforts while diagnostic interventions confirm and classify lesion severity. Within screening, cytology remains a foundational tool, yet HPV testing has surged to the forefront by offering predictive value across a wider genotype spectrum, and visual inspection with acetic acid retains relevance for point-of-care scenarios. Diagnostic pathways rely heavily on biopsy and colposcopy, where enhanced imaging and sampling precision are critical for guiding treatment decisions. Therapeutic modalities, including cryotherapy, electrosurgical excision, and laser ablation, each cater to specific lesion grades and clinical settings, underpinning the market’s emphasis on personalized care.
From a product-type vantage, the market spans biopsy forceps and colposcopes to advanced device categories such as cryotherapy units, electrosurgical hardware, endocervical curettes, and laser systems. This breadth highlights the interplay between foundational surgical tools and cutting-edge energy-based solutions. End-user segmentation differentiates between ambulatory surgical centers and specialty clinics, where speed and workflow integration are paramount, and hospitals along with diagnostic laboratories-both hospital based and independent-where throughput and analytical rigor dominate. Understanding these cross-segment dynamics is essential for stakeholders aiming to align product development and commercialization strategies with evolving clinical and operational imperatives.
This comprehensive research report categorizes the Cervical Dysplasia market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- End User
Comprehensive regional examination showcasing unique regulatory, infrastructure, and adoption trends across the Americas, Europe, Middle East & Africa, and Asia-Pacific
Regional insights illustrate a geographically diversified market for cervical dysplasia solutions, with the Americas maintaining leadership through robust healthcare infrastructure and proactive public health programs targeting HPV vaccination and screening adherence. Latin America is emerging as a high-growth arena, supported by initiatives to expand access in community clinics and government-subsidized screening pathways. In Europe, Middle East & Africa, heterogeneous regulatory environments and varied reimbursement frameworks challenge suppliers to tailor their market entry strategies, while pan-regional efforts to standardize clinical guidelines are fostering harmonized screening protocols. North Africa and parts of the Gulf region are witnessing incremental adoption of portable cryotherapy devices as part of broader women’s health initiatives.
The Asia-Pacific region stands out for its dynamic innovation ecosystem, where collaborations between private hospitals and diagnostic laboratories are driving early adoption of AI-enabled cytology and multiplex HPV assays. In Southeast Asia and parts of Oceania, telecolposcopy platforms are gaining traction, bridging gaps in specialist availability and optimizing referral networks. These regional distinctions underscore the importance of localized market intelligence, emphasizing that successful market penetration strategies must account for differences in clinical practice, reimbursement dynamics, and infrastructure maturity across global markets.
This comprehensive research report examines key regions that drive the evolution of the Cervical Dysplasia market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Advanced strategic movements and partnerships illustrate how industry leaders are integrating device innovation with digital platforms to reinforce market dominance
A competitive landscape analysis highlights the strategic maneuvers of leading device and diagnostic companies as they vie for market share in the cervical dysplasia domain. Major players are investing heavily in research collaborations to expand their assay portfolios, focusing on multi-genotype HPV testing to enhance clinical utility. Concurrently, several medical technology companies are partnering with contract manufacturers to scale production of biopsy instruments and energy-based treatment devices, ensuring supply continuity amidst geopolitical trade uncertainties.
In addition, technology vendors are launching integrated digital platforms that unify case management across screening, diagnostics, and treatment. These ecosystems enable real-time data exchange between ambulatory surgical centers, specialty clinics, hospitals, and diagnostic laboratories. Strategic acquisitions are also reshaping the competitive terrain, with several firms seeking to bolster their end-user penetration by adding portable cryotherapy systems or advanced colposcopic imaging to their product suites. Overall, market leaders are differentiating through innovation ecosystems that span device hardware, software analytics, and comprehensive service models designed to elevate procedural efficiency and patient outcomes.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cervical Dysplasia market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Arbor Vita Corporation
- Asieris Pharmaceuticals
- ASKA Pharmaceutical Co., Ltd.
- Becton, Dickinson, and Company
- Bio-Rad Laboratories, Inc.
- Bristol-Myers Squibb Company
- Cardinal Health Inc.
- CooperSurgical Inc
- DYSIS Medical Ltd.
- F. Hoffmann La-Roche Ltd
- GlaxoSmithKline PLC
- Guided Therapeutics, Inc.
Strategic recommendations encourage cross-sector collaborations, localized manufacturing, and AI-powered analytics to advance comprehensive cervical dysplasia management
Industry leaders should prioritize forging cross-sector alliances that integrate diagnostics, device manufacturing, and data analytics to establish holistic care pathways. By aligning cytology and HPV testing providers with colposcopic imaging specialists, stakeholders can co-develop bundled solutions that reduce administrative burden and enhance clinical adoption rates. Additionally, accelerating the rollout of portable cryotherapy and electrosurgical units in community settings will not only expand market reach but also support global health initiatives by increasing procedural accessibility.
Another critical recommendation is to explore localized manufacturing partnerships to mitigate tariff-related cost pressures and ensure resilient supply chains. Engaging with both hospital-based and independent diagnostic laboratories on reagent standardization can streamline onboarding of new assays and harmonize quality controls. Finally, deploying AI-driven analytics across end-user segments can uncover inefficiencies in referral patterns and treatment workflows, enabling continuous process optimization and reinforcing value-based care objectives.
Rigorous research methodology combining primary interviews, secondary data validation, and scenario analysis ensures robust insights into market realities
This study synthesizes primary insights from in-depth interviews with clinical key opinion leaders and procurement decision makers across hospitals, ambulatory surgical centers, specialty clinics, and diagnostic laboratories. Secondary sources, including peer-reviewed publications and regulatory filings, were meticulously reviewed to validate clinical guidelines and device approval timelines. Custom surveys were deployed to quantify adoption drivers for screening and treatment modalities, ensuring a robust synthesis of qualitative and quantitative inputs.
To enhance data integrity, triangulation methods were applied, cross-referencing procurement trends with sales data from device manufacturers and assay providers. Detailed vendor profiling included evaluations of R&D pipelines, patent filings, and partnership announcements. The methodology also integrated scenario analysis to assess the impact of regulatory changes and tariff implementations on supply-chain dynamics. This rigorous approach ensures that the findings reflect the most current market realities and provide actionable intelligence for strategic decision making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cervical Dysplasia market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cervical Dysplasia Market, by Product Type
- Cervical Dysplasia Market, by Application
- Cervical Dysplasia Market, by End User
- Cervical Dysplasia Market, by Region
- Cervical Dysplasia Market, by Group
- Cervical Dysplasia Market, by Country
- United States Cervical Dysplasia Market
- China Cervical Dysplasia Market
- Competitive Landscape
- List of Figures [Total: 15]
- List of Tables [Total: 1272 ]
Synthesis of key insights underscores the intersection of technological, regulatory, and regional dynamics that will shape future cervical dysplasia solutions
In summary, the cervical dysplasia market is poised at a critical junction where technological advancements, regulatory shifts, and regional dynamics converge to redefine screening and treatment paradigms. The increasing integration of multiplex HPV assays and AI-enabled cytology is enhancing diagnostic precision, while innovations in cryotherapy, electrosurgical excision, and laser ablation are elevating therapeutic outcomes. Although new tariffs have introduced cost complexities, they have also catalyzed opportunities for domestic manufacturing expansions and supply-chain optimization.
Stakeholders equipped with nuanced segmentation intelligence and localized market understanding will be well positioned to capitalize on emerging growth corridors. By embracing open innovation frameworks and targeted partnerships, industry leaders can deliver comprehensive cervical dysplasia solutions that improve patient pathways and align with global health objectives. As the market evolves, continuous adaptation and strategic foresight will be paramount in maintaining competitive advantage and delivering value to patients and providers alike.
Secure tailored cervical dysplasia market intelligence and strategic guidance from our Associate Director of Sales & Marketing to drive informed decision making
To gain the most comprehensive insights into the evolving cervical dysplasia market and to secure a competitive edge in your strategic planning, reach out to Ketan Rohom (Associate Director, Sales & Marketing). Engage today to receive a personalized briefing that delves into granular segmentation analyses, regional momentum, and tariff impacts. By partnering directly, you can customize your deliverables to align with your organization’s objectives, ensuring your investments in diagnostic, screening, and treatment technologies are guided by the most current intelligence. Our experts stand ready to support you with tailored data interrogations, deeper dive workshops, and executive presentations. Don’t miss this opportunity to leverage exclusive foresight and position yourself for growth as the cervical dysplasia landscape undergoes pivotal transformations.

- How big is the Cervical Dysplasia Market?
- What is the Cervical Dysplasia Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




