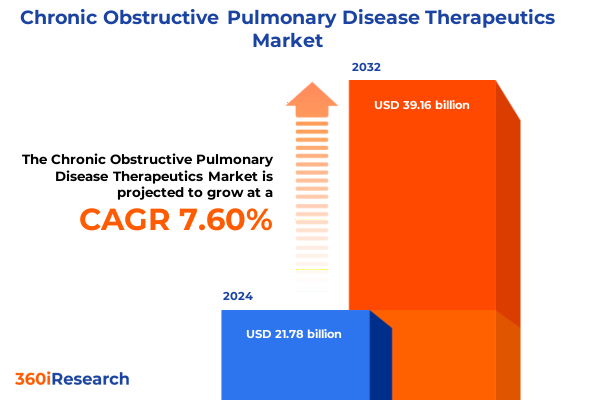
The Chronic Obstructive Pulmonary Disease Therapeutics Market size was estimated at USD 23.41 billion in 2025 and expected to reach USD 25.16 billion in 2026, at a CAGR of 7.62% to reach USD 39.16 billion by 2032.
Unveiling the critical landscape of COPD therapeutics and highlighting emerging imperatives shaping patient outcomes and market evolution
Chronic Obstructive Pulmonary Disease (COPD) remains a critical public health challenge, imposing significant morbidity, mortality, and economic burden across global healthcare systems. Recent years have witnessed the convergence of clinical innovation and regulatory evolution, driving new therapeutic modalities aimed at improving lung function, reducing exacerbations, and enhancing quality of life for patients. At the same time, demographic shifts such as aging populations and rising prevalence of environmental risk factors are reshaping patient cohorts and intensifying demand for personalized treatment approaches.
Moreover, the competitive environment is more dynamic than ever before. Established pharmaceutical players are contending with the emergence of biotech innovators and digital health pioneers, each introducing novel inhalation devices, combination therapies, and precision medicine tools. In tandem with evolving clinical guidelines, these developments are converging to redefine the standard of care. Consequently, understanding the multi-dimensional forces at work-from scientific breakthroughs and competitive repositioning to shifting patient expectations-is paramount for stakeholders seeking to unlock value and drive sustainable growth in this high-stakes arena.
Examining groundbreaking advancements and paradigm shifts revolutionizing chronic obstructive pulmonary disease treatment modalities worldwide
Over the past decade, transformative shifts have radically altered the COPD therapeutic ecosystem. Breakthrough research into dual and triple inhaled combination therapies has delivered clinically meaningful improvements in lung function and exacerbation reduction, setting new benchmarks for standard of care. These pharmacological advances are being complemented by innovative inhalation technologies-dry powder inhalers, soft-mist inhalers, and advanced nebulizer systems-that enhance drug delivery efficiency and patient adherence.
Furthermore, the integration of digital health platforms and smart inhaler sensors is revolutionizing disease management by capturing real-time adherence and lung function data. This data-driven paradigm fosters more proactive, patient-centric care models and enables the tailoring of treatment regimens based on individual response patterns. Meanwhile, the growing emphasis on preventive healthcare has stimulated interest in early-intervention strategies, including novel mucolytics and anti-inflammatory agents that aim to slow disease progression.
Consequently, market participants must adapt swiftly to these shifts, aligning R&D pipelines, manufacturing capabilities, and commercial strategies with the accelerating pace of scientific progress and evolving stakeholder expectations.
Assessing how the 2025 United States tariffs on pharmaceutical imports are reshaping COPD treatment cost structures and supply chain dynamics
In 2025, newly implemented United States tariffs on imported active pharmaceutical ingredients and specialized inhalation devices have introduced material shifts in cost structures, pricing strategies, and supply chain resilience for COPD therapeutics. Manufacturers that rely heavily on overseas sourcing have faced rising input costs, compelling them to reassess supplier agreements and logistics frameworks. As a result, several companies have expedited efforts to diversify procurement-seeking alternate sites in Mexico and select domestic manufacturing partnerships to mitigate tariff exposure.
Moreover, the tariffs have triggered strategic price negotiations between payers and manufacturers, with managed care organizations demanding greater transparency around cost pass-throughs. This dynamic has fueled value-based contracting pilots, where reimbursement is increasingly linked to real-world patient outcomes such as exacerbation reduction and hospitalization avoidance. In parallel, some market leaders have absorbed portions of the tariff burden to preserve competitive price positioning, prioritizing long-term market share gains over short-term margin impacts.
Consequently, the cumulative impact of the 2025 US tariffs underscores the necessity for agile supply chain strategies, innovative contracting approaches, and robust stakeholder engagement to sustain access and affordability in the evolving COPD therapeutic landscape.
Delving into nuanced therapeutic product categories, administration routes, distribution channels, and end-user landscapes informing COPD treatment strategies
COPD therapeutic strategies are best understood through a multifaceted lens of product and service segmentation. Across antibiotic courses, bronchodilator regimens, novel combination products, inhaled corticosteroids, and supportive mucolytic agents, treatment paradigms are increasingly customized to patient-specific pharmacological needs and comorbidity profiles. Moreover, the route of administration exerts a profound influence on clinical efficacy and patient adherence; inhalation modalities remain dominant, yet injectable biologics and oral small molecules continue to carve out critical niches, particularly when formulation innovations enhance bioavailability or minimize systemic exposure.
Within inhalation, the relative adoption of dry powder inhalers, metered dose inhalers, and advanced nebulizer platforms is shaped by factors such as device ergonomics, dose consistency, and patient dexterity. Distribution channels further diversify patient access pathways: hospital pharmacies continue to serve acute care needs, while online pharmacies and retail outlets deliver convenience-focused solutions for maintenance therapy. Finally, end users ranging from specialized respiratory clinics to homecare nursing services and hospital outpatient departments drive tailored support offerings, including patient education, remote monitoring, and adherence coaching. By synthesizing these segmentation dimensions, stakeholders can refine go-to-market models, optimize product positioning, and pinpoint unmet needs across the COPD continuum.
This comprehensive research report categorizes the Chronic Obstructive Pulmonary Disease Therapeutics market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product
- Route Of Administration
- Distribution Channel
- End User
Comparative analysis of COPD therapeutic dynamics across the Americas, Europe Middle East Africa, and Asia Pacific regions revealing distinctive trends
Regional dynamics in COPD therapeutics exhibit distinctive patterns driven by healthcare infrastructure, regulatory frameworks, and epidemiological profiles. In the Americas, robust reimbursement systems and a strong emphasis on value-based care have accelerated the uptake of combination inhalers and digital adherence tools, while market access negotiations frequently hinge on pharmacoeconomic evidence and real-world outcomes.
Conversely, in Europe Middle East Africa, heterogeneous regulatory pathways and variable payer environments necessitate regionally tailored market entry and pricing approaches. Countries with centralized health technology assessment bodies demand rigorous clinical and economic dossiers, whereas emerging markets in the region are increasingly attractive for manufacturers willing to navigate complex import regulations and forge local partnerships to ensure supply continuity.
Meanwhile, Asia Pacific presents a dual narrative: developed economies within the region are fostering advanced biologic therapies and telemedicine-driven management programs, whereas developing nations contend with infrastructure constraints and prioritize cost-effective generics and fixed-dose combinations. Through a comparative lens, these regional insights reveal where R&D prioritization, commercial tactics, and collaborative models can be adapted to capitalize on local market nuances and growth opportunities.
This comprehensive research report examines key regions that drive the evolution of the Chronic Obstructive Pulmonary Disease Therapeutics market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling leading innovators and established pharmaceutical companies that are driving competitive differentiation in COPD therapeutic development
Leading pharmaceutical and biotech organizations are actively shaping the COPD treatment frontier through differentiated pipelines and strategic collaborations. Established global players have leveraged decades of respiratory expertise to introduce next-generation combination inhalers, while nimble midsize innovators are concentrating on targeted biologics and novel anti-inflammatory molecules. Concurrently, several technology-driven firms have partnered with device manufacturers to launch connected inhalation systems, layering remote patient monitoring capabilities onto traditional drug delivery.
These companies are also tapping into strategic alliances with contract development and manufacturing organizations to accelerate scale-up and manage cost efficiencies. Licensing agreements and co-promotion deals have become essential levers, enabling access to proprietary formulations and distribution networks across key markets. In addition, venture-backed entities specializing in inhaled gene therapy and nanoparticle-based mucus modulators are emerging as potential disruptors, reflecting the intense race to pioneer truly disease-modifying interventions.
By tracking these competitive and collaborative dynamics, stakeholders can anticipate potential shifts in market leadership, identify partnership prospects, and align their strategic roadmaps with forward-looking therapeutic strategies.
This comprehensive research report delivers an in-depth overview of the principal market players in the Chronic Obstructive Pulmonary Disease Therapeutics market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Almirall S.A.
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca plc
- Aurobindo Pharma Ltd.
- Boehringer Ingelheim International GmbH
- Chiesi Farmaceutici S.p.A
- Cipla Limited
- CSL Limited
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Ltd.
- Lupin Ltd.
- Mylan N.V.
- Novartis AG
- Orion Corporation
- Sandoz International GmbH
- Sun Pharmaceutical Industries Ltd
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd
- Theravance Biopharma, Inc.
- Verona Pharma plc
- Yuhan Corporation
Strategic imperatives and best practice recommendations empowering industry leaders to optimize COPD therapeutic portfolios and patient engagement
To maintain a competitive edge in the dynamic COPD sector, industry leaders should prioritize strategic initiatives that align with evolving clinical needs and market realities. First, diversifying supply chains through regional manufacturing hubs and nearshoring can mitigate tariff-induced cost pressures and enhance resilience against geopolitical disruptions. Second, embracing value-based contracting frameworks will strengthen payer relationships by demonstrating tangible patient outcomes linked to inhaled therapies and support services.
In addition, advancing digital health integrations-such as smart inhalers, telemonitoring platforms, and AI-driven adherence interventions-can foster deeper patient engagement and generate longitudinal real-world evidence. Coupling these technologies with comprehensive patient support programs offers an integrated care model that improves clinical success and drives brand loyalty. Moreover, investing in next-generation combination formulations and novel biologic candidates remains critical; prioritizing assets with robust differentiation and clear differentiation across efficacy, safety, and convenience profiles will fortify pipeline pipelines.
Finally, forging strategic partnerships along the value chain-from device innovators to commercial distributors-can unlock synergies and accelerate market penetration. Through these actionable recommendations, industry leaders can future-proof their COPD portfolios and sustainably elevate patient care standards.
Outlining rigorous research design combining primary insights, secondary analysis, and expert validation underpinning the COPD therapeutics study
This COPD therapeutics analysis is founded upon a rigorous research design that blends primary and secondary methodologies to ensure robust, actionable insights. Primary research entailed in-depth interviews with key opinion leaders, pulmonologists, payers, and patient advocacy groups across major markets, providing firsthand perspectives on treatment patterns, unmet needs, and adoption barriers.
Complementing these qualitative insights, secondary research involved systematic reviews of peer-reviewed journals, regulatory filings, clinical trial registries, conference proceedings, and publicly available reimbursement databases. Data triangulation techniques were applied to validate findings, reconcile discrepancies, and enhance the reliability of trend projections.
Furthermore, expert validation workshops were conducted with cross-functional stakeholders-ranging from R&D heads and market access specialists to supply chain leaders-to refine strategic recommendations and corroborate segmentation assumptions. This multi-layered methodology underpins the credibility of reported insights and equips decision-makers with a transparent, evidence-based foundation to guide strategic planning and investment decisions.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Chronic Obstructive Pulmonary Disease Therapeutics market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Chronic Obstructive Pulmonary Disease Therapeutics Market, by Product
- Chronic Obstructive Pulmonary Disease Therapeutics Market, by Route Of Administration
- Chronic Obstructive Pulmonary Disease Therapeutics Market, by Distribution Channel
- Chronic Obstructive Pulmonary Disease Therapeutics Market, by End User
- Chronic Obstructive Pulmonary Disease Therapeutics Market, by Region
- Chronic Obstructive Pulmonary Disease Therapeutics Market, by Group
- Chronic Obstructive Pulmonary Disease Therapeutics Market, by Country
- United States Chronic Obstructive Pulmonary Disease Therapeutics Market
- China Chronic Obstructive Pulmonary Disease Therapeutics Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 954 ]
Summarizing key takeaways and underscoring the imperative for collaborative innovation in addressing the evolving challenges of COPD care
In summary, the COPD therapeutics landscape is experiencing unprecedented transformation driven by novel combination therapies, advanced inhalation technologies, and emerging digital health solutions. These shifts are further shaped by geopolitical developments such as the 2025 US tariffs, which have accentuated the importance of supply chain agility and innovative contracting models.
By dissecting market segmentation across product classes, administration routes, distribution channels, and end users, stakeholders can craft highly targeted strategies that resonate with specific patient and payer requirements. Regional comparisons highlight where tailored market entry and commercialization approaches can unlock growth, while profiling key industry players provides clarity on competitive dynamics and partnership opportunities.
As the field continues to evolve, embracing data-driven decision-making, collaborative innovation, and proactive stakeholder engagement will be vital for delivering improved patient outcomes and sustaining commercial success. This analysis offers an authoritative guide to navigating the complexities of COPD therapeutics and capitalizing on the opportunities that lie ahead.
Engage with Ketan Rohom for exclusive access to a comprehensive COPD therapeutics market insights report tailored to executive decision-makers
In today s dynamic COPD treatment environment, understanding the full suite of therapeutic insights is essential. Connect with Ketan Rohom, an experienced associate director specializing in sales and marketing strategy, to gain privileged access to a comprehensive COPD therapeutics report tailored for influential decision-makers. By engaging directly, stakeholders can secure forward-looking analyses, deep dives into segmented market dynamics, and actionable recommendations that will inform growth strategies across product portfolios and geographic regions.

- How big is the Chronic Obstructive Pulmonary Disease Therapeutics Market?
- What is the Chronic Obstructive Pulmonary Disease Therapeutics Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




