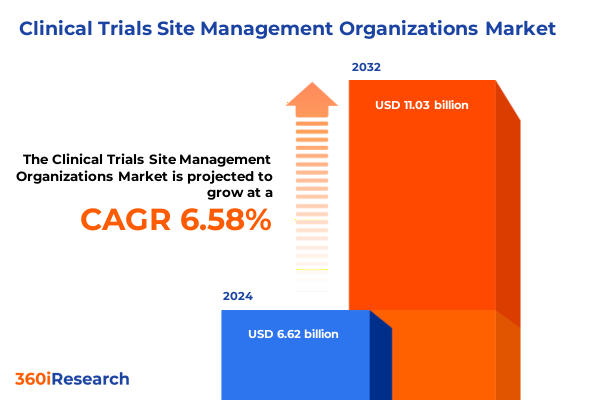
The Clinical Trials Site Management Organizations Market size was estimated at USD 7.04 billion in 2025 and expected to reach USD 7.49 billion in 2026, at a CAGR of 6.62% to reach USD 11.03 billion by 2032.
Discover how clinical trial site management organizations are navigating complex regulatory environments, embracing digital transformation, and optimizing operational workflows to accelerate study timelines and enhance research quality
Within an era of escalating complexity in clinical research, site management organizations have emerged as indispensable partners, orchestrating every dimension of trial execution. The convergence of stringent regulatory standards, rapid technological advancements, and heightened expectations for patient safety has necessitated a new level of diligence in site operations. Consequently, sponsors and investigators now rely on specialized SMOs to navigate intricate compliance requirements, optimize resource allocation, and ensure seamless coordination across dispersed trial locations.
As clinical protocols grow in sophistication, the role of the SMO extends far beyond administrative oversight. These organizations deploy comprehensive strategies that integrate digital tools, patient engagement frameworks, and real-time performance analytics to enhance trial efficiency. By centralizing site activation, recruitment, and monitoring functions, SMOs drive consistency in data quality and operational discipline. This streamlined approach not only mitigates trial delays but also cultivates a robust foundation for adaptive study designs.
Looking forward, the proliferation of decentralized and hybrid trials will further amplify the significance of site management expertise. Organizations that master the interplay between virtual care models and traditional site interactions will gain a distinct advantage, positioning themselves at the forefront of clinical innovation.
Unveiling pivotal transformations driving the evolution of site management in clinical trials through decentralization, advanced analytics, patient centricity, and integrated end-to-end service delivery
The landscape of site management is undergoing a profound metamorphosis, driven by a convergence of technological breakthroughs and evolving trial paradigms. Decentralized trial models have emerged as a cornerstone of this transformation, empowering patients to participate through telemedicine consultations and remote monitoring devices. By decentralizing data collection and expanding virtual touchpoints, SMOs are redefining the boundaries of geographic reach and patient diversity.
Simultaneously, the integration of advanced analytics into trial operations has unlocked new dimensions of predictive site performance. Machine learning algorithms sift through historical and real-world data to forecast recruitment bottlenecks, optimize resource allocation, and prioritize high-yield sites. These data-driven insights enable site management leaders to deploy targeted interventions early, ensuring that studies remain on schedule even in the face of unforeseen challenges.
Moreover, the rise of patient centricity has reshaped site engagement strategies. SMOs now collaborate closely with advocacy groups and community stakeholders to co-design recruitment campaigns and retention programs that resonate with target populations. This human-centric approach, combined with risk-based monitoring frameworks, has enhanced patient safety oversight while reducing on-site visits. As these transformative shifts gain momentum, industry leaders must align their operations with hybrid execution models, balancing virtual and on-site activities to unlock both efficiency and participant satisfaction.
Assessing the far-reaching effects of United States tariffs implemented in 2025 on clinical trial site management operations, supply chains, and cross-border collaborations
In 2025, the imposition of new United States tariffs on imported clinical supplies and equipment introduced a ripple effect across site management operations. Increasing duties on electronic monitoring devices and laboratory reagents have eroded traditional cost structures, compelling SMOs to revisit supplier agreements and reengineer procurement strategies. As a result, organizations have accelerated diversification of vendor portfolios, seeking domestic partners and regional distributors to mitigate the impact of elevated import costs.
The tariff environment has also prompted a reassessment of cross-border collaborations. Trials with multinational footprints have encountered challenges in harmonizing budgets across regions with disparate cost escalations. In response, site management professionals have adopted dynamic budgeting frameworks that account for real-time currency fluctuations and duty adjustments. This agile approach ensures that study progress remains uninterrupted, even as external trade policies evolve.
Furthermore, the cumulative effect of these tariffs has underscored the importance of resilient supply chain management. SMOs are leveraging integrated inventory tracking systems to maintain just-in-time stock levels, reducing both excess holding costs and the risk of trial delays due to material shortages. By embedding tariff impact analysis into financial forecasting, site management teams can now forecast cost contingencies with greater precision. This strategic alignment of procurement, finance, and operational planning not only safeguards trial continuity but also reinforces the agility needed to thrive under shifting trade landscapes.
Gaining strategic vision through segmentation insights on service offerings, trial phases, technology adoption, and end user demands shaping site management dynamics
A nuanced understanding of segmentation reveals critical pathways to optimizing site management strategies within distinct service types, trial phases, technology integrations, and end user requirements. Service offerings range from clinical monitoring and patient recruitment to project management, regulatory affairs, and site selection and activation. Each service dimension demands tailored workflows, yet collectively they form an interlocking ecosystem that underpins trial success.
Equally, site management maturities differ across Phase I to Phase IV studies, necessitating bespoke operational frameworks. Early-phase trials prioritize tight safety oversight and rapid protocol amendments, whereas late-stage studies require robust patient retention programs and expansive data management capacities. Recognizing these phase-specific nuances allows SMOs to allocate specialized teams and resources in alignment with the evolving demands of each trial stage.
Technology solutions further stratify the market, as clinical trial management systems, electronic data capture platforms, and patient engagement applications converge to drive digital efficiency. The orchestration of these tools within a unified technology stack enhances real-time visibility into site performance metrics and participant adherence trends. Finally, end users-including biotechnology firms, contract research organizations, medical device companies, and pharmaceutical manufacturers-each bring unique priorities around speed, compliance, and therapeutic expertise. By synthesizing segmentation insights across these four dimensions, site management leaders can craft targeted strategies that amplify operational impact and drive competitive differentiation.
This comprehensive research report categorizes the Clinical Trials Site Management Organizations market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Service Type
- Phase
- Technology Solutions
- End User
Exploring distinctive regional dynamics across the Americas, EMEA, and Asia-Pacific to illuminate regulatory frameworks, operational challenges, cultural considerations, and stakeholder engagement strategies
Regional dynamics exert a profound influence on site management practices, as varying regulatory frameworks, cultural considerations, and market maturity levels shape trial execution. In the Americas, a robust infrastructure for clinical research and streamlined regulatory pathways support rapid site activation and patient recruitment. Leading markets in North America, in particular, benefit from established investigator networks and advanced digital ecosystems that enable hybrid trial models.
Conversely, Europe, the Middle East, and Africa present a complex mosaic of regulatory nuances and market access barriers. Compliance with GDPR and region-specific health authority requirements demands meticulous planning, while collaborative partnerships with local institutions are essential to navigate fragmented approval processes. At the same time, the expansion of clinical research hubs in Eastern Europe and parts of the Middle East underscores the region’s potential as a growth frontier.
Asia-Pacific has emerged as a critical growth engine for global trials, offering cost-effective site networks and rapidly evolving regulatory harmonization initiatives. Countries such as Japan, South Korea, and Australia have advanced frameworks for expedited reviews, while emerging markets across Southeast Asia are investing in clinical research infrastructure. By calibrating strategies to regional strengths and aligning stakeholder engagement models with local norms, SMOs can unlock heightened efficiency and foster deeper community trust in each territory.
This comprehensive research report examines key regions that drive the evolution of the Clinical Trials Site Management Organizations market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting leading site management organizations reshaping clinical research through innovation, data-driven platforms, global networks, and specialized expertise across diverse therapeutic areas
The competitive landscape of clinical trial site management is shaped by organizations distinguished by their global footprint, technology integration, and therapeutic expertise. A cohort of leading SMOs has leveraged proprietary data analytics platforms to optimize site selection, augment patient recruitment, and mitigate operational risks. Their investment in virtual site monitoring and decentralized trial infrastructure has enabled sponsors to expand access to otherwise underserved patient populations.
Concurrently, companies with integrated diagnostics and laboratory capabilities have differentiated themselves by offering seamless end-to-end services. This integration streamlines data flow between sample collection and analysis, reduces reconciliation timelines, and enhances overall study quality. In parallel, niche players specializing in complex therapeutic areas such as oncology, rare diseases, and advanced biologics have attracted sponsors seeking tailored site management solutions that reflect deep domain knowledge.
Emerging entrants focused on patient engagement technologies and community outreach programs have also influenced market dynamics. By embedding mobile applications, digital consent processes, and remote monitoring tools, these innovators have redefined standards for participant retention. Taken together, these varied strategic approaches underscore the importance of aligning organizational strengths with specific trial objectives to foster competitive advantage in an increasingly sophisticated SMO ecosystem.
This comprehensive research report delivers an in-depth overview of the principal market players in the Clinical Trials Site Management Organizations market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Accel Clinical Services
- Accelagen
- Altasciences
- Celerion
- ClinChoice
- Clinical Development Solutions
- CMIC HOLDINGS Co., LTD.
- Criterium, Inc.
- FIDELIS RESEARCH AD by BioIVT
- FOMAT Medical Research Inc.
- George Clinical Pty Ltd
- Grand Pacific CRO
- ICON PLC
- IQVIA Inc.
- L.E.K. Consulting LLC
- Medigence Solutions Pvt Ltd.
- Novotech Health Holdings
- Parexel International Corporation
- PPD Inc. by Thermo Fisher Scientific Inc.
- PROMETRIKA, LLC.
- ProTrials Research, Inc.
- Red Maple Trials Inc.
- SGS S.A.
- Veristat, LLC.
- Vial Health Technology, Inc.
- WIRB-Copernicus Group
- Xylem Research LLP
- Zave Clinical Research Management
Implementing targeted recommendations that enable industry leaders to optimize site performance, streamline digital integration, and foster collaborative partnerships for sustainable growth
To thrive in the rapidly evolving landscape of site management, industry leaders must adopt a multi-pronged approach that balances innovation, operational excellence, and strategic collaboration. First, embedding advanced analytics into every facet of trial execution allows organizations to predict and preempt challenges before they escalate. By harnessing real-time performance data, site management teams can dynamically adjust recruitment tactics, resource allocation, and monitoring frequencies to sustain optimal study progress.
Second, investing in integrated digital platforms that unify CTMS, EDC, and patient engagement functionalities will drive both efficiency and transparency. A consolidated technology ecosystem not only accelerates data flow but also enhances visibility for sponsors, investigators, and regulatory stakeholders. Equally, fostering strategic partnerships with specialized vendors and local research networks will reinforce supply chain resilience and expand access to targeted patient cohorts.
Furthermore, elevating patient-centric initiatives through community engagement and tailored retention programs will differentiate site performance metrics. Prioritizing participant convenience and trust, from streamlined consent processes to hybrid visit schedules, yields higher adherence and data integrity. Ultimately, embedding these recommendations within a continuous improvement framework will empower site management organizations to achieve sustainable growth and maintain leadership in a competitive global marketplace.
Describing the rigorous research methodology combining primary interviews, secondary data analysis, and comprehensive validation to ensure robust insights and credibility
This research report draws upon a comprehensive methodology designed to capture both breadth and depth of market insights. Initially, an extensive secondary research phase reviewed peer-reviewed publications, regulatory guidelines, industry white papers, and public financial disclosures to establish a robust foundational understanding. Concurrently, primary research interviews were conducted with senior executives, clinical operations leaders, and site management specialists to capture real-world perspectives and emerging best practices.
Data triangulation played a central role in validating findings, ensuring that qualitative insights were reinforced by quantitative metrics. Proprietary databases and external analytics platforms were leveraged to cross-verify trends in site performance, patient enrollment rates, and technology adoption. This iterative validation process included feedback loops with an expert advisory panel, whose members represent leading SMOs, pharmaceutical sponsors, and regulatory consultants.
Quality assurance protocols were maintained throughout, with periodic reviews for data consistency and methodological rigor. Adherence to established research standards and ethical guidelines ensured transparency and objectivity. The culmination of these efforts provides a credible and actionable framework for understanding the evolving site management landscape and guiding strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Clinical Trials Site Management Organizations market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Clinical Trials Site Management Organizations Market, by Service Type
- Clinical Trials Site Management Organizations Market, by Phase
- Clinical Trials Site Management Organizations Market, by Technology Solutions
- Clinical Trials Site Management Organizations Market, by End User
- Clinical Trials Site Management Organizations Market, by Region
- Clinical Trials Site Management Organizations Market, by Group
- Clinical Trials Site Management Organizations Market, by Country
- United States Clinical Trials Site Management Organizations Market
- China Clinical Trials Site Management Organizations Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 795 ]
Concluding insights underscore the imperative for flexible, technology-enabled, patient-centric, and resilient site management strategies to navigate evolving clinical trial landscapes and stakeholder expectations
The collective insights presented in this report highlight the imperative for site management organizations to remain agile, technologically adept, and patient-focused. As trial paradigms continue to embrace decentralization and hybrid models, operational frameworks must evolve to incorporate virtual engagement channels alongside traditional on-site activities. Technology will serve as both the enabler and differentiator, with advanced analytics and integrated platforms driving efficiency and transparency.
Moreover, the shifting landscape of global trade policies and regional regulatory variances underscores the need for resilient supply chains and dynamic budgeting strategies. SMOs that proactively adapt procurement processes and foster collaborative partnerships across borders will mitigate external risks and sustain study momentum. Finally, a deep appreciation for segmentation and regional nuances will guide targeted service offerings that resonate with diverse stakeholders, from biotech innovators to established pharmaceutical enterprises.
By weaving together these strategic considerations, industry leaders can position their organizations to navigate complexity, deliver high-quality trial outcomes, and foster enduring partnerships. The path forward demands a balance of innovation, operational discipline, and unwavering commitment to patient safety and data integrity. In doing so, site management organizations will continue to play a pivotal role in advancing clinical research and bringing novel therapies to patients worldwide.
Take decisive action today by reaching out to Ketan Rohom to secure comprehensive market research insights that will drive your strategic site management decisions
To explore the full breadth of insights on clinical trials site management organizations-spanning transformative trends, regional dynamics, and actionable best practices-connect directly with Ketan Rohom, Associate Director of Sales & Marketing. Ketan can guide you through tailored research packages that align with your strategic objectives for site performance optimization and clinical innovation.
By engaging with Ketan Rohom, you will gain immediate access to in-depth analyses, comparative profiles of leading SMOs, and forward-looking recommendations designed to address your unique operational challenges. His expertise in translating market intelligence into operational roadmaps will empower your organization to make informed investment decisions and strengthen stakeholder collaborations.
Reach out to initiate a personalized briefing or request a comprehensive proposal outlining how this market research report can support your site management strategies. Partner with an expert who understands the nuances of clinical trial execution and is committed to delivering clarity and direction for your most critical research initiatives.

- How big is the Clinical Trials Site Management Organizations Market?
- What is the Clinical Trials Site Management Organizations Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




