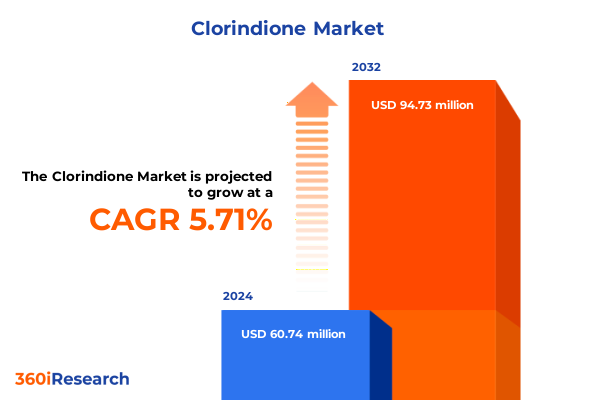
The Clorindione Market size was estimated at USD 64.04 million in 2025 and expected to reach USD 68.05 million in 2026, at a CAGR of 5.75% to reach USD 94.73 million by 2032.
Unveiling Clorindione’s Role in Modern Anticoagulation Through its Mechanisms, Clinical Applications, and Regulatory Considerations
Clorindione is a vitamin K antagonist belonging to the coumarin derivative class, recognized for its efficacy in preventing and treating thromboembolic disorders through targeted inhibition of the vitamin K epoxide reductase complex. By disrupting the regeneration of reduced vitamin K, Clorindione effectively reduces the synthesis of key clotting factors II, VII, IX, and X, thereby exerting its anticoagulant effect that is critical in managing deep vein thrombosis, pulmonary embolism, and in reducing stroke risk among patients with atrial fibrillation. Clinicians administering Clorindione must carefully monitor patients’ International Normalized Ratio to balance therapeutic benefit against bleeding risk, adapting dosage based on individual metabolic response and concurrent therapies to ensure optimal outcomes and patient safety
Navigating the Paradigm Shift in Anticoagulant Therapy Driven by Direct Oral Anticoagulants and Digital Health Innovations
The anticoagulant landscape has undergone a profound transformation with the advent of direct oral anticoagulants (DOACs), which now rival traditional vitamin K antagonists such as Clorindione due to their predictable pharmacokinetics and reduced requirement for routine monitoring. Devices and digital platforms that enable remote patient monitoring, medication reminders, and virtual consultations are increasingly integral to anticoagulation management, fostering higher adherence rates and safer therapeutic regimens. Concurrently, pharmaceutical manufacturers have been refining DOAC formulations-such as extended-release rivaroxaban and simplified apixaban dosing-to enhance convenience for patients with chronic cardiovascular conditions and to expand indication profiles beyond non-valvular atrial fibrillation, further intensifying competition within the anticoagulant market. As telehealth integration continues to rise, anticoagulant therapies are shifting toward patient-centric models that leverage real-time data analytics, enabling clinicians to proactively adjust treatment and mitigate adverse events, thereby redefining the standard of care in thromboembolic disease management.
Assessing the Aggregate Impact of 2025 United States Tariffs on Pharmaceutical Ingredients and Anticoagulant Supply Chains
In April 2025, the United States implemented a 10% global tariff on nearly all imports, encompassing active pharmaceutical ingredients essential to the production of anticoagulants like Clorindione. This measure has amplified input costs for drugmakers relying on foreign-sourced intermediates, particularly impacting generics with thin margins and putting pressure on the entire supply chain. Moreover, escalating trade tensions have prompted proposals for tariffs up to 25% on finished pharmaceutical products, which could elevate U.S. drug expenditures by an estimated $51 billion annually if passed through to consumers, increasing prescription costs by as much as 12.9% and potentially exacerbating shortages of critical medications. Experts warn that such duties on imported active ingredients would not only raise production costs but also undermine domestic competitiveness, delaying any potential resurgence in onshore manufacturing due to the lengthy regulatory and infrastructure investments required to establish new facilities.
Decoding Clorindione Market Dynamics Through Formulation, Application, End Use, and Distribution Channel Perspectives
The Clorindione market analysis delves into multiple dimensions, beginning with formulation, where the drug’s effectiveness, stability profiles, and patient adherence considerations are examined across capsule, liquid, and tablet presentations, each tailored to diverse clinical and patient preferences. Equally important is the distinction between prophylaxis and treatment applications; while prophylactic use focuses on long-term prevention of thrombotic events, the therapeutic segment addresses acute management of deep vein thrombosis and pulmonary embolism. Demand is further stratified by end-use environments-ambulatory surgical centers benefit from rapid-onset protocols, home care services rely on user-friendly regimens, and hospitals require robust supply and monitoring systems for critical care patients. Finally, distribution channels influence market reach, encompassing hospital pharmacies that serve inpatient needs, online pharmacies that cater to remote refills and telehealth models, and retail pharmacies offering community access and point-of-care consultation.
This comprehensive research report categorizes the Clorindione market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Formulation
- Distribution Channel
- Application
- End Use
Unearthing Regional Insights: Strategic Trends in the Americas, Europe Middle East & Africa, and Asia-Pacific Markets for Clorindione
In the Americas, a mature healthcare infrastructure and high prevalence of cardiovascular diseases drive consistent demand for established anticoagulants, while evolving regulatory frameworks and ongoing tariffs challenge stakeholders to optimize supply chains and localize production. Transitioning to digital health solutions has improved adherence, yet cost pressures from potential duties necessitate strategic sourcing and value-based contracting. In Europe, Middle East & Africa, robust pharmaceutical R&D hubs in Western Europe and growing healthcare investments across the Middle East are expanding access to both generic and novel anticoagulants, with jurisdictional differences in reimbursement policies influencing adoption patterns and regional partnerships. Meanwhile, the Asia-Pacific region exhibits the fastest growth trajectory, fueled by government support for cardiovascular disease management, rising patient awareness, and expanding hospital networks in China, India, and Southeast Asia, all underpinned by the integration of point-of-care testing and mobile health platforms that streamline anticoagulation management.
This comprehensive research report examines key regions that drive the evolution of the Clorindione market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Illuminating Competitive Landscape and Innovation Pathways Among Key Clorindione and Anticoagulant Market Players
The competitive environment for Clorindione is shaped by niche manufacturers such as Berlin-Chemie, which maintains production expertise in phenindione derivatives and supplies the drug under established trade names, positioning itself in specialized anticoagulant portfolios. Surrounding this core, leading global pharmaceutical companies-including Bayer, Bristol-Myers Squibb, Pfizer, AstraZeneca, and Johnson & Johnson-are investing heavily in next-generation anticoagulant R&D, digital management tools, and expansive manufacturing footprints within the United States to preempt tariff risks and address evolving clinical guidelines. Strategic alliances between API suppliers and contract developers are also emerging to ensure consistent raw material availability, while pharmaceutical distributors are integrating telehealth-friendly services to deliver anticoagulant therapies seamlessly across patient care settings.
This comprehensive research report delivers an in-depth overview of the principal market players in the Clorindione market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Alfa Chemistry by Thermo Fisher Scientific Inc.
- BASF SE
- Bayer AG
- Bell Laboratories, Inc.
- Biosynth AG
- Crescent Chemical Co., Inc.
- FMC Corporation
- Liphatech, LLC
- Merck & Co., Inc.
- Neogen Corporation
- Nufarm Limited
- PelGar International Limited
- Sumitomo Chemical Co., Ltd.
- Syngenta International AG
Strategic Playbook of Actionable Recommendations to Enhance Clorindione Market Resilience and Growth
Industry leaders should prioritize diversifying active ingredient sourcing to mitigate tariff exposure by establishing strategic partnerships with suppliers across multiple regions and exploring in-licensing opportunities for alternative vitamin K antagonist compounds. Embracing digital health technologies can strengthen patient adherence and safety monitoring for Clorindione, leveraging mobile platforms and remote diagnostics to differentiate offerings. Companies are advised to engage with regulatory bodies proactively to navigate evolving trade policies and secure tariff exemptions for critical intermediates. Additionally, expanding presence in high-growth markets through localized manufacturing collaborations and tailored patient support programs will foster resilience and drive long-term uptake in both established and emerging geographies. Finally, aligning with value-based reimbursement models can demonstrate real-world efficacy and cost offsets, reinforcing Clorindione’s role amid a competitive anticoagulant portfolio.
Rigorous Research Methodology Leveraging Primary Interviews, Secondary Analysis, and Data Triangulation for Market Insights
This analysis synthesizes insights derived from a multi-stage research framework. Primary data was collected through structured interviews with hematologists, pharmacists, and supply chain executives, providing firsthand perspectives on Clorindione usage and procurement challenges. Concurrently, secondary research encompassed peer-reviewed literature, regulatory filings, clinical trial registries, and proprietary databases such as DrugBank and KEGG, ensuring technical accuracy regarding pharmacological properties and therapeutic applications. Quantitative data on tariff impacts and broader anticoagulant market trends were triangulated against reports from reputable financial and policy analysts. All findings underwent rigorous validation through cross-referencing with industry experts and market participants to ensure reliability and relevance to strategic decision-makers.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Clorindione market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Clorindione Market, by Formulation
- Clorindione Market, by Distribution Channel
- Clorindione Market, by Application
- Clorindione Market, by End Use
- Clorindione Market, by Region
- Clorindione Market, by Group
- Clorindione Market, by Country
- United States Clorindione Market
- China Clorindione Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 795 ]
Distilling Key Findings to Highlight Clorindione’s Strategic Imperatives and Future Outlook in Anticoagulant Therapies
Clorindione remains a critical vitamin K antagonist within the anticoagulant spectrum, characterized by established clinical applications and a well-understood safety profile. Yet, transformative shifts driven by the rise of DOACs, digital health integration, and evolving tariff landscapes necessitate strategic adaptation. Segmentation analysis highlights differentiated demand across formulations and care settings, while regional insights underscore both mature market stability and high-growth potential in emerging economies. Key industry players are actively innovating through R&D investments and supply chain realignments, presenting collaboration and competitive opportunities for Clorindione stakeholders. Ultimately, success will hinge on agile sourcing strategies, proactive regulatory engagement, and leveraging technology to optimize patient outcomes and maintain market relevance.
Connect with Ketan Rohom to Unlock Comprehensive Clorindione Market Intelligence and Empower Strategic Decision-Making
For further details on acquiring the comprehensive market research report on Clorindione’s evolving landscape, please reach out to Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch, who can guide you through the insights and facilitate your purchase. Engage with him to explore tailored data, in-depth analyses, and strategic recommendations that will empower your organization to navigate the complex dynamics of anticoagulant therapies.

- How big is the Clorindione Market?
- What is the Clorindione Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




