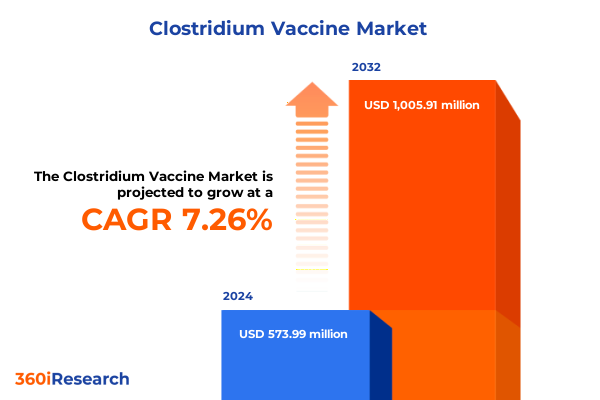
The Clostridium Vaccine Market size was estimated at USD 613.79 million in 2025 and expected to reach USD 660.20 million in 2026, at a CAGR of 7.31% to reach USD 1,005.91 million by 2032.
Introducing the Critical Role of Vaccines Targeting Clostridium Diseases Amid Rising Threats and Innovative Protection Strategies
The escalating incidence of diseases caused by Clostridium species has sharply underscored the critical need for effective prophylactic measures. This introduction lays the foundation by highlighting how botulism, gas gangrene, and tetanus continue to impose severe public health burdens across developed and emerging economies alike. Modern healthcare systems are grappling with an uptick in antimicrobial resistance and sporadic outbreak clusters, prompting renewed focus on vaccine-based prevention strategies. As a result, stakeholders spanning government agencies, pharmaceutical developers, and global health organizations are accelerating efforts to bring next-generation Clostridium Vaccines to market.
Beyond the immediate epidemiological threats, advances in immunological science have opened new avenues for improving vaccine efficacy and safety. Conjugate platforms, recombinant subunit designs, and refined toxoid formulations represent a paradigm shift away from traditional approaches. These scientific breakthroughs, when paired with evolving distribution infrastructures, are poised to reshape how public health systems deploy preventive care. In light of this dynamic environment, the following report sections will delve into transformative industry shifts, tariff impacts, and nuanced market segmentation to provide a thorough executive summary of the Clostridium Vaccine market landscape.
Emerging Technological Breakthroughs and Policy Synergies Are Redefining the Global Clostridium Vaccine Development Ecosystem
In recent years, the Clostridium Vaccine landscape has witnessed transformative accelerations driven by technological innovation and strategic partnerships. Recombinant Vaccine platforms have matured, enabling scalable manufacturing of highly purified antigen components, while conjugate approaches-both oligosaccharide-conjugated and protein-conjugated-have demonstrated enhanced immunogenicity, particularly among vulnerable populations. Concurrently, the integration of advanced adjuvant systems has optimized immune responses, reducing dosage requirements and facilitating broader immunization campaigns.
Policy initiatives have also played a pivotal role in reshaping the competitive terrain. Public-private partnerships have unlocked funding for late-stage clinical programs targeting tetanus and gas gangrene, while regulatory harmonization efforts seek to streamline cross-border vaccine approvals. Digital health technologies, including cold-chain monitoring and electronic health record integration, are enhancing distribution efficiency, increase accountability, and bolstering patient adherence. Together, these disruptive forces are converging to redefine how Clostridium Vaccines are researched, approved, produced, and delivered globally.
Analysis of How the 2025 United States Trade Measures Have Reshaped Supply Chain Dynamics and Cost Structures for Clostridium Vaccine Production
The imposition of United States tariffs in early 2025 introduced a complex layer of cost considerations for Clostridium Vaccine manufacturers. Across antigen component imports, particularly specialized conjugate precursors sourced from select overseas biotech suppliers, cumulative duties have exerted upward pressure on upstream manufacturing expenditures. This has prompted vaccine developers to reassess global supply chains and identify domestic or tariff-exempt sourcing alternatives to maintain competitive price points.
In parallel, higher input costs have accelerated dialogues around localizing production capacity within the United States. Several leading vaccine producers have initiated feasibility studies for onshore fermentation facilities and fill-finish centers to insulate their operations from tariff volatility. Meanwhile, strategic alliances are emerging as key mitigation tactics, with U.S. biotech firms forging partnerships with international contract development and manufacturing organizations to secure tariff-efficient supply channels. These adaptive responses underscore how evolving trade policies are shaping investment decisions and competitive positioning across the Clostridium Vaccine market.
Insights into Platform Diversity, Disease-Specific Delivery, and Distribution Pathways Driving Clostridium Vaccine Uptake
The Clostridium Vaccine arena is characterized by nuanced segmentation that influences both development priorities and market delivery models. Vaccine Type segmentation encompasses conjugate technologies-distinguished further into oligosaccharide-conjugated and protein-conjugated variants-alongside polysaccharide-based formulations, recombinant subunit vaccines, and classic toxoid approaches. This diversity of platforms addresses heterogeneous immunogenic profiles, catering to adult, pediatric, and at-risk groups with differentiated immune responses.
To target specific Clostridium-related conditions, the market segregates by disease focus, spanning botulism, gas gangrene, and tetanus immunization needs, each with unique antigenic requirements and clinical adoption pathways. Route of Administration trends reveal intradermal delivery gaining traction for dose-sparing strategies, while established intramuscular and subcutaneous injections remain prevalent in most immunization programs. Distribution channels range from hospital pharmacies and retail chains to burgeoning online pharmacies, which encompass both broad e-commerce marketplaces and direct manufacturer websites seeking to capture digital-first patient segments. Finally, end-user segmentation identifies ambulatory care centers, clinics, hospitals-with private and public hospital settings-and public health centers as primary vaccination points, each demanding tailored logistical and engagement approaches.
This comprehensive research report categorizes the Clostridium Vaccine market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Vaccine Type
- Target Disease
- Route Of Administration
- Distribution Channel
- End User
Comparative Analysis of Regional Adoption Patterns and Infrastructure Capabilities in Shaping Vaccine Access Globally
Geographically, the Clostridium Vaccine market exhibits distinct characteristics across the Americas, Europe Middle East and Africa, and Asia-Pacific regions. In the Americas, robust immunization infrastructure and supportive reimbursement frameworks have catalyzed high adoption of advanced conjugate and recombinant vaccine types. Collaborative agreements between government agencies and domestic biotech developers are driving localized clinical trials and pilot immunization programs.
Within Europe Middle East and Africa, regulatory harmonization initiatives led by regional bodies have facilitated streamlined approvals, yet heterogeneous healthcare funding models pose challenges for uniform market access. Strategic partnerships between multinational vaccine producers and regional manufacturers are emerging to navigate divergent public procurement processes. Conversely, in Asia-Pacific, rising healthcare expenditures, expanding cold-chain networks, and strong public health mandates are accelerating uptake of intradermal and intramuscular Clostridium Vaccines, particularly in densely populated urban centers. Across all three macro-regions, tailored distribution strategies and localized R&D collaborations are essential to address distinct epidemiological profiles and patient engagement preferences.
This comprehensive research report examines key regions that drive the evolution of the Clostridium Vaccine market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Assessment of Collaboration Strategies and Technology Platforms Driving Competitive Advantage Among Top Clostridium Vaccine Developers
The competitive landscape of the Clostridium Vaccine market is defined by established pharmaceutical incumbents alongside agile biotech innovators. Major global players have leveraged integrated R&D pipelines that span antigen discovery to commercial manufacturing, harnessing proprietary adjuvants and formulation expertise to extend product lifecycles. At the same time, emerging biotech firms are differentiating through niche recombinant vaccine platforms and targeted clinical programs focusing on gas gangrene and botulism prophylaxis.
Strategic collaborations and licensing agreements have become hallmarks of market leadership, enabling technology transfers that accelerate time to market. Several alliances between biotech start-ups and multinational vaccine producers have yielded co-development programs for next-generation toxoid conjugates. Meanwhile, contract development and manufacturing organizations are expanding their service offerings to support intensified fill-finish demand. As cost-containment pressures mount, competitive dynamics will hinge on the ability to balance innovative payloads with scalable, tariff-resilient supply chains.
This comprehensive research report delivers an in-depth overview of the principal market players in the Clostridium Vaccine market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Bayer AG
- Bharat Biotech International Limited
- Bimeda, Inc.
- Biogénesis Bagó S.A.
- Cadila Healthcare Limited
- Ceva Santé Animale S.A.
- China National Biotec Group Co., Ltd.
- Colorado Serum Company
- Elanco Animal Health Incorporated
- GlaxoSmithKline plc
- Hester Biosciences Limited
- Huvepharma Inc.
- IDT Biologika GmbH
- Laboratorios Hipra S.A.
- Merck & Co., Inc.
- Panacea Biotec Limited
- Pfizer Inc.
- Phibro Animal Health Corporation
- Sanofi S.A.
- Tianjin Ringpu Biotechnology Co., Ltd.
- Vaxxinova International B.V.
- Vetoquinol S.A.
- Virbac S.A.
- Zoetis Services LLC
Strategic Blueprints for Building Resilient Manufacturing Networks and Advancing Clinical Innovations in the Clostridium Vaccine Arena
Industry leaders should prioritize investment in modular manufacturing facilities that can flexibly switch between vaccine platforms, ensuring resilient response capabilities amid tariff and supply chain uncertainties. Deepening partnerships with raw material suppliers-particularly for conjugate precursors-will be critical to securing cost-stable procurement pathways. Equally important is the expansion of digital distribution channels, with enhanced e-commerce platforms and direct-to-clinic delivery models tailored to evolving healthcare consumer behaviors.
On the scientific front, advancing combination approaches that integrate toxoid and recombinant antigens could unlock broader-spectrum immunization, while novel adjuvant systems offer pathways to dose reduction and cost-efficiency. Engaging in proactive regulatory dialogue at both national and regional levels will streamline approval processes, particularly for innovative administration routes such as intradermal dosing. Finally, establishing localized public health alliances in emerging markets will foster stakeholder alignment and accelerate immunization campaign uptake.
Rigorous Qualitative and Quantitative Methodology Ensuring Robust Segmentation and Validated Competitive Analysis
Our research methodology integrates a multi-tiered approach combining primary qualitative interviews with subject-matter experts, vaccine developers, and procurement officers, alongside exhaustive secondary research of peer-reviewed literature, patent filings, and regulatory databases. Competitive benchmarking was conducted through analysis of recent product approvals, clinical trial registries, and company annual disclosures, ensuring a holistic view of pipeline dynamics and market entry timelines.
To validate findings, a triangulation process cross-referenced revenue reports, technology licensing announcements, and manufacturing capacity data. Segmentation frameworks were refined through workshops with industry advisory panels, aligning vaccine platform categorizations, target disease priorities, and distribution channel definitions with current market practice. Regional insights were corroborated via dialogues with public health authorities and logistic service providers across the Americas, Europe Middle East and Africa, and Asia-Pacific, ensuring a grounded perspective on access challenges and infrastructural capabilities.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Clostridium Vaccine market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Clostridium Vaccine Market, by Vaccine Type
- Clostridium Vaccine Market, by Target Disease
- Clostridium Vaccine Market, by Route Of Administration
- Clostridium Vaccine Market, by Distribution Channel
- Clostridium Vaccine Market, by End User
- Clostridium Vaccine Market, by Region
- Clostridium Vaccine Market, by Group
- Clostridium Vaccine Market, by Country
- United States Clostridium Vaccine Market
- China Clostridium Vaccine Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1431 ]
Summarizing Market Drivers and Strategic Imperatives Defining the Future Trajectory of Clostridium Vaccine Development
This executive summary has elucidated the multifaceted forces shaping the Clostridium Vaccine market, from technological breakthroughs and trade policy impacts to nuanced segmentation and regional adoption disparities. The convergence of innovative conjugate, recombinant, and toxoid platforms heralds a new era of preventive solutions against botulism, gas gangrene, and tetanus, while global tariff shifts underscore the urgency of adaptable supply chain strategies.
Competitive positioning will be determined by the agility to form strategic alliances, localize manufacturing, and harness digital distribution channels. As public health imperatives intensify, the capacity to deliver safe, effective, and accessible Clostridium Vaccines will rely on integrated R&D, resilient partnerships, and proactive engagement with regulatory frameworks. Such coordinated efforts will define the next chapter in safeguarding populations from Clostridium-related diseases worldwide.
Unlock Authoritative Clostridium Vaccine Insights by Connecting Directly with the Associate Director of Sales and Marketing for Tailored Market Intelligence
To explore comprehensive insights into the Clostridium Vaccine market and how strategic decisions can reshape your organization’s competitive trajectory, reach out to Ketan Rohom, Associate Director of Sales & Marketing. With a deep understanding of both the scientific innovations and market dynamics fueling this critical healthcare segment, Ketan is uniquely positioned to guide you toward the actionable intelligence needed for confident decision-making. Engage directly to secure a tailored copy of the full research report, leveraging granular analysis on pipeline developments, regulatory landscapes, and commercialization strategies. Connect now to equip your team with the most current, in-depth market intelligence and gain a strategic advantage in the evolving Clostridium Vaccine landscape.

- How big is the Clostridium Vaccine Market?
- What is the Clostridium Vaccine Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




