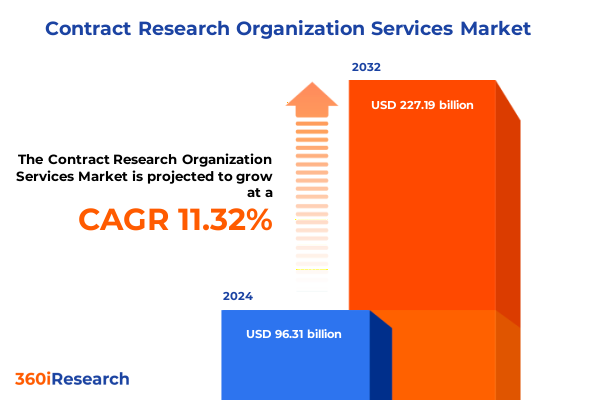
The Contract Research Organization Services Market size was estimated at USD 83.45 billion in 2025 and expected to reach USD 87.43 billion in 2026, at a CAGR of 6.94% to reach USD 133.54 billion by 2032.
Setting the stage for insights into contract research organization services transforming clinical and laboratory operations worldwide
The evolving landscape of contract research organization services is reshaping how pharmaceutical and biotechnology companies manage clinical development, laboratory operations, and regulatory compliance. This executive summary lays the groundwork for understanding the key dimensions influencing service delivery and strategic decision-making. Over the following pages, we introduce the strategic context, examine market transformations, and highlight insights that can guide stakeholders in optimizing their engagement with research partners.
In this introduction, we define the primary service domains encompassed by clinical trial management, laboratory services, and regulatory consulting, each of which plays a critical role in accelerating program timelines and ensuring data integrity. We also outline how tariff fluctuations, segmentation diversity, and regional dynamics intersect to create both challenges and opportunities for service providers and sponsors alike. Through this lens, readers will appreciate the interconnected nature of operational efficiencies, cost structures, and compliance standards.
Finally, we preview how the subsequent sections unpack transformative trends, analyze the impact of recent policy shifts, delineate actionable segmentation and regional insights, profile leading industry participants, and offer strategic recommendations. This structured approach equips decision-makers with a cohesive narrative and clear reference points to evaluate potential partnerships, allocate resources effectively, and anticipate future market developments.
Examining unprecedented digital innovation and regulatory realignments driving transformative shifts in contract research service delivery models
The contract research industry is undergoing a wave of transformative shifts driven by digital innovation, evolving regulatory demands, and shifting sponsor expectations. Advancements in decentralized trial platforms and remote monitoring technologies have accelerated the adoption of virtual and hybrid study designs, enabling sponsors to access more diverse patient populations and streamline data collection processes. Moreover, real-time data analytics, powered by artificial intelligence and machine learning, are optimizing patient enrollment predictions and risk-based monitoring, thereby reducing operational bottlenecks and improving trial outcomes.
Simultaneously, regulatory agencies are issuing new guidance on electronic data capture, data integrity, and cybersecurity, prompting service providers to enhance their compliance infrastructures. Across the laboratory services spectrum, digital assay platforms and automated workflows are elevating throughput capacity while ensuring rigorous quality control. In parallel, the rise of biomarker-driven drug development is intensifying collaboration between bioanalysis specialists and clinical teams, underscoring the need for integrated service models that facilitate seamless information flow.
Furthermore, the convergence of real-world evidence with traditional clinical trial data is fostering more robust regulatory submissions and evidence packages. As sponsors increasingly demand end-to-end service integration, contract research organizations are aligning cross-functional capabilities to deliver synchronized project management, centralized lab operations, and strategic regulatory support. These developments signal a paradigm shift from discrete service offerings to holistic, technology-enabled partnerships.
Analyzing the cumulative ramifications of recent United States tariff policies on contract research service cost structures and operational frameworks in 2025
The introduction of new United States tariff measures in early 2025 has engendered a cumulative impact on cost structures and procurement strategies across contract research service providers. By extending Section 301 tariffs to laboratory equipment imports and specialized reagents, service providers have encountered import cost increases of up to 25 percent. Consequently, procurement teams are diversifying their supplier base, forging strategic alliances with domestic vendors, and negotiating volume-based agreements to mitigate input cost volatility.
Moreover, tariff-induced cost pressures have prompted a reevaluation of pricing models and contract terms, with sponsors seeking greater transparency in cost breakdowns and service level commitments. This shift has accelerated the adoption of value-based contracting and risk-sharing arrangements, ensuring that sponsors and providers collaboratively manage budgetary impacts. Regional service hubs in North America have also witnessed increased demand, as sponsor organizations pivot resources toward domestic laboratories to bypass import tariffs and maintain operational continuity.
Furthermore, providers with vertically integrated laboratory networks have gained a competitive edge by consolidating sourcing and centralizing manufacturing activities. By leveraging in-house reagent production and equipment maintenance capabilities, these organizations have absorbed tariff burdens while preserving margin profiles. Taken together, these dynamics underscore the necessity for agile supply chain strategies and adaptive contracting frameworks to navigate an increasingly complex economic environment.
Revealing granular insights across multiple segmentation lenses that illuminate actionable pathways for contract research stakeholders
The diverse segmentation landscape of contract research services illuminates where strategic focus can unlock maximum value. Through the lens of service types, the clinical trial management domain-spanning data management, project management, and site management-demonstrates how integrated oversight accelerates study timelines. Conversely, laboratory services, encompassing bioanalysis, biomarker development, and central lab operations, reveal opportunities for vertical integration to optimize throughput and quality control. Parallel to this, regulatory consulting services, which include compliance auditing, regulatory strategy development, and submission management, emphasize the growing importance of specialized expertise in navigating evolving global regulatory frameworks.
In the therapeutic arena, cardiovascular disease programs such as coronary artery disease and heart failure studies require precise endpoint adjudication and robust data modeling, while infectious disease trials tackling bacterial and viral pathogens are accelerating parallel assay development and supply chain coordination. Oncology research, divided between hematologic malignancies and solid tumors, highlights the need for complex biomarker panels and adaptive design methodologies. Examining the phases of development reveals unique challenges at each stage: dose escalation and first-in-human trials in Phase I demand nimble safety monitoring; Phase II dose optimization and efficacy analyses depend on advanced statistical modeling; Phase III confirmatory trials require comprehensive medical monitoring; and preclinical animal studies and in vitro experiments set the foundational proof of concept.
End users ranging from academic research institutes and universities to both innovative and generic biotechnology firms, as well as large pharmaceutical enterprises and small to medium-sized companies, illustrate the spectrum of service expectations, from educational collaborations to fully bespoke program execution. Finally, contract duration preferences-whether long-term partnerships under strategic alliances and therapeutic area collaborations or short-term multi-study and single-study contracts-shape resource allocation and capacity planning. Together, these segmentation insights guide service providers in tailoring solutions to sponsor priorities and research imperatives.
This comprehensive research report categorizes the Contract Research Organization Services market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Service Types
- Therapeutic Areas
- Phases of Development
- Contract Periods
- End Users
Uncovering differentiated regional dynamics shaping contract research demand across Americas, EMEA, and Asia-Pacific markets
Regional dynamics across the Americas underscore the preeminence of North America as a hub for clinical research, driven by established regulatory bodies, robust healthcare infrastructure, and significant R&D investment. Sponsors in this region often favor integrated service models that align project management, data analytics, and laboratory capabilities under a single contract, streamlining communication and accelerating timelines. In contrast, Latin American markets are emerging as attractive sites for cost-efficient patient recruitment and decentralized trial pilots, with growing local CRO networks facilitating complex study logistics.
Within Europe, Middle East & Africa, diverse regulatory landscapes from the European Medicines Agency’s stringent guidance to region-specific health authority requirements necessitate specialized compliance consulting and adaptive study design. The region’s strategic access to varied patient populations enables oncology and rare disease programs to demonstrate extensive safety and efficacy data. Meanwhile, Asia-Pacific markets present robust growth potential, fueled by large patient pools, competitive operating costs, and expanding clinical infrastructure, particularly in biopharma innovation clusters across China, Japan, and Southeast Asia. A surge in capacity expansion and favorable government incentives continues to attract global sponsors seeking operational efficiency and geographic diversification.
By understanding the differentiated demands, regulatory complexities, and growth trajectories within each region, service providers can strategically allocate resources, prioritize service enhancements, and forge partnerships that capitalize on local strengths while maintaining global consistency.
This comprehensive research report examines key regions that drive the evolution of the Contract Research Organization Services market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting leading contract research organizations setting benchmarks through innovation, strategic alliances, and service diversification
A select group of contract research organizations has differentiated itself by combining service breadth, technological innovation, and global reach. Leading service providers are investing heavily in digital platforms that standardize data capture across trial sites and integrate real-world evidence streams to enhance asset characterization. Concurrently, strategic alliances-such as joint ventures with specialized laboratories and technology firms-are extending service portfolios to include next-generation sequencing, advanced biomarker assays, and decentralized trial support.
Furthermore, mergers and acquisitions continue to reshape the competitive landscape, with high-profile deals expanding geographic footprints and bolstering domain expertise in fields like gene therapy and precision oncology. Providers that have maintained a clear focus on quality metrics, regulatory compliance, and client satisfaction metrics are witnessing stronger renewal rates and increased share-of-wallet opportunities. At the same time, companies that prioritize thought leadership-through white papers, expert panels, and real-time data dashboards-are fostering deeper client engagement and reinforcing their reputational advantages.
As contract research organizations navigate the balance between scale and specialization, those that effectively align technology-enabled service models with strategic partnerships will be best positioned to meet evolving sponsor needs and capture incremental growth in high-value service segments.
This comprehensive research report delivers an in-depth overview of the principal market players in the Contract Research Organization Services market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AccuLab Life Sciences
- ACTIVA-CRO
- Advanced Clinical Research Services, LLC
- BioAgile Therapeutics Pvt. Ltd.
- Caidya
- Calian Group Ltd.
- Celerion, Inc.
- Charles River Laboratories International, Inc.
- Clinical Trial Service B.V. by PCM Trials
- Cromsource by ClinChoice
- CTI Clinical Trial & Consulting
- Distefar del Sur SL
- Ergomed PLC
- Firma Clinical Research, LLC
- Frontage Holdings Corporation
- Geistek Pharma S.L.
- HCL Technologies Limited
- Icon PLC
- INQUIS
- IQVIA Holdings Inc.
- KCR S.A.
- Laboratory Corporation of America Holdings
- León Research, S.L.
- Linical Co., Ltd.
- Medpace Holdings, Inc.
- Novotech Health Holdings
- OPIS S.r.l.
- Oxon Epidemiology, S.L.
- Parexel International Corporation
- Pepgra
- Pharmaron Beijing Co., Ltd.
- Pivotal, S.L.U.
- PPD Inc. by Thermo Fisher Scientific Inc.
- Prometrika, LLC
- ProRelix Services LLP
- PSI CRO AG
- QualitecFarma S.L.
- SGS S.A.
- Syncro Clinical Research SRL
- Syneos Health, Inc.
- The Emmes Company, LLC
- Veeda Clinical Research Limited
- Vial Health Technology, Inc.
- Worldwide Clinical Trials Holdings Inc.
- WuXi AppTec Co., Ltd.
- X7 Research
Empowering industry leaders with strategic imperatives to navigate evolving market dynamics and accelerate competitive differentiation
Industry leaders must embrace a multipronged approach to capitalize on evolving market dynamics and sustain competitive differentiation. First, prioritizing investment in digital trial management platforms-leveraging artificial intelligence for patient recruitment, monitoring, and adaptive analytics-will streamline workflows and reduce time-to-data. In addition, expanding decentralized trial capabilities through partnerships with technology aggregators and telehealth providers can enhance patient engagement and broaden geographic reach, while maintaining data integrity and compliance.
Second, diversifying service portfolios to include emerging modalities such as advanced cell and gene therapy support and comprehensive real-world evidence offerings will capture growth in high-margin segments. Collaborating with academic centers and niche biotechs to co-develop specialized assays or therapeutic area modules can further enrich value propositions. Moreover, strengthening regional hubs-particularly in Latin America and Asia-Pacific-through targeted capacity investments and local regulatory expertise will enable sponsors to navigate diverse markets efficiently.
Finally, implementing agile contracting frameworks that balance risk-sharing and performance-based incentives will align sponsor-provider interests and facilitate transparent pricing structures. By adopting these strategic imperatives, contract research organizations can drive operational excellence, foster long-term customer partnerships, and secure sustainable growth in a rapidly shifting landscape.
Outlining a rigorous mixed-methods approach integrating primary expert insights and comprehensive secondary analysis for robust market intelligence
Our research methodology combines rigorous secondary research, primary interviews, and quantitative validation to deliver a comprehensive perspective on the contract research organization services market. Initially, publicly available sources-including peer-reviewed journals, regulatory guidance documents, industry white papers, and reputable financial disclosures-were analyzed to identify foundational trends, service innovations, and policy shifts. This secondary phase established a robust knowledge base upon which further investigation was built.
Building on these insights, expert interviews were conducted with senior executives, clinical operations directors, regulatory affairs specialists, and procurement leads at both sponsor organizations and contract research providers. These conversations probed real-world challenges, emerging best practices, and anticipated future priorities, ensuring that our analysis reflects current operational realities. Data triangulation techniques were applied to reconcile varying perspectives and validate qualitative findings with quantitative metrics derived from recent service activity reports and project deliverables.
Finally, our segmentation framework was stress-tested through scenario analysis to assess the interplay between service types, therapeutic areas, development phases, end-user profiles, and contract durations. This methodological rigor guarantees that the insights presented in this report are not only analytically sound but also highly relevant for stakeholders seeking to optimize their partnerships and strategic initiatives.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Contract Research Organization Services market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Contract Research Organization Services Market, by Service Types
- Contract Research Organization Services Market, by Therapeutic Areas
- Contract Research Organization Services Market, by Phases of Development
- Contract Research Organization Services Market, by Contract Periods
- Contract Research Organization Services Market, by End Users
- Contract Research Organization Services Market, by Region
- Contract Research Organization Services Market, by Group
- Contract Research Organization Services Market, by Country
- United States Contract Research Organization Services Market
- China Contract Research Organization Services Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 3339 ]
Synthesizing critical findings into a cohesive narrative illustrating the future trajectory of contract research organization services
The synthesis of our analysis paints a clear picture: contract research organizations must adapt to technological disruption, regulatory evolution, and macroeconomic pressures to maintain their strategic relevance. Digital transformation and decentralized trial methodologies are no longer optional-they are imperative for accelerating program timelines and enhancing data quality. Simultaneously, the ripple effects of U.S. tariff policies have underscored the value of supply chain resilience and adaptive contracting practices.
Our segmentation examination reveals that tailoring service offerings to specific therapeutic areas, development phases, and end-user requirements can unlock new revenue streams and deepen client engagements. Regional comparisons show that a nuanced approach-balancing global consistency with local adaptability-drives operational excellence and market penetration. Moreover, the competitive landscape continues to favor organizations that invest in innovative partnerships, service integration, and quality-focused execution.
In conclusion, the future trajectory of contract research services hinges on an organization’s ability to synthesize these cross-cutting insights into cohesive strategic initiatives. By leveraging the recommendations outlined earlier, stakeholders can forge resilient partnerships, optimize resource allocation, and chart a course toward sustained growth.
Driving immediate action through a personalized invitation to access advanced market research solutions tailored for meaningful business outcomes
If you’re ready to leverage comprehensive insights that can sharpen your competitive edge, connect with Ketan Rohom, Associate Director, Sales & Marketing. He can guide you through the full suite of detailed analyses and tailor the market research report to address your organization’s most pressing questions. By initiating a collaborative conversation today, you’ll gain the clarity and confidence needed to make evidence-based decisions that drive sustained growth. Reach out to explore available customization options, discuss pricing tiers, and schedule a personalized briefing session to translate these findings into a strategic action plan.

- How big is the Contract Research Organization Services Market?
- What is the Contract Research Organization Services Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




