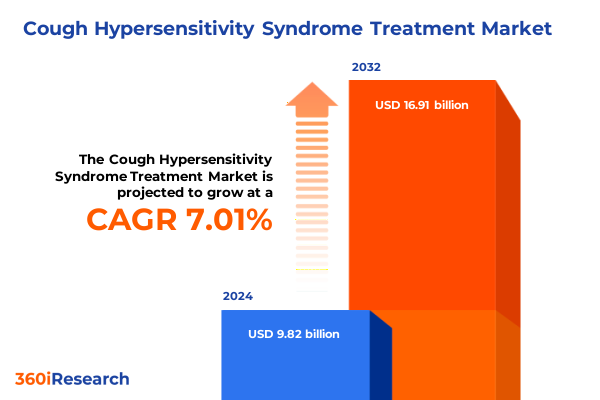
The Cough Hypersensitivity Syndrome Treatment Market size was estimated at USD 10.41 billion in 2025 and expected to reach USD 11.03 billion in 2026, at a CAGR of 7.17% to reach USD 16.91 billion by 2032.
Unraveling the Complexities of Cough Hypersensitivity Syndrome Treatment: A Foundational Overview of Evolving Therapeutic Strategies
Cough hypersensitivity syndrome (CHS) represents a complex clinical entity characterized by a persistent, unrelenting cough that lacks an identifiable underlying cause despite exhaustive evaluation. Patients often describe an exaggerated response to innocuous stimuli-cold air, perfumes, or even talking-that triggers debilitating cough episodes, significantly impairing daily function and quality of life. Among adults presenting to specialized clinics for refractory chronic cough, the prevalence of CHS has risen in parallel with growing recognition of neural reflex hypersensitivity as a central pathophysiological driver.
The evolving understanding of CHS positions it not merely as a symptom but as a treatable trait within broader airway and respiratory disorders. Groundbreaking work has reframed CHS through the lens of cough reflex sensitivity, prompting clinicians and researchers to explore pharmacological and nonpharmacological strategies that address both central and peripheral neural mechanisms. This paradigm shift is underpinned by data demonstrating the efficacy of targeted interventions such as P2X3 receptor antagonists and behavioral cough suppression therapy.
As the therapeutic landscape expands, stakeholders across healthcare systems-from clinicians to payers-are increasingly focused on precision management approaches that transcend traditional antitussives. With unmet clinical needs remaining substantial, a comprehensive overview of CHS treatment modalities is essential for guiding research priorities, investment decisions, and patient care pathways in this rapidly advancing field.
How Pioneering Pharmaceutical Innovations and Nonpharmacological Approaches Are Redefining Patient Outcomes in Chronic Cough Management
In recent years, the management of chronic cough associated with CHS has undergone a transformative evolution fueled by both pharmaceutical innovation and reconceptualized nonpharmacological interventions. Once limited to conventional antitussive agents, the therapeutic arsenal now encompasses neuromodulatory drugs such as gabapentin and pregabalin, which modulate neural excitability in central pathways, offering symptomatic relief for patients unresponsive to first-line therapies.
Simultaneously, the emergence of peripheral-targeted therapies has redefined the treatment paradigm. Selective P2X3 receptor antagonists, most notably gefapixant, have demonstrated the capacity to attenuate aberrant cough reflex sensitivity by inhibiting purinergic signaling on airway afferent nerves, heralding a new era of mechanism-based antitussives. Although taste disturbances remain a dose-limiting side effect, ongoing clinical development and optimization of dosing regimens hold promise for broader adoption upon regulatory approval.
Complementing pharmacological advances, nonpharmacological strategies-particularly speech and language therapy–driven cough suppression techniques-have garnered robust evidence supporting their integration into comprehensive CHS management programs. By focusing on laryngeal hygiene, voluntary cough control, and psychoeducational counseling, these interventions address higher cortical and behavioral contributors to cough hypersensitivity, further diversifying the treatment landscape and improving long-term patient outcomes.
Assessing the Ripple Effects of the 2025 United States Tariff Regime on Pharmaceutical Ingredients and Medical Device Accessibility
Beginning in early 2025, a broad 10% global tariff on most imported goods entering the United States has introduced significant cost pressures across the healthcare supply chain. Among the most impacted items are active pharmaceutical ingredients (APIs), which underpin the production of both generic and branded cough treatments. The tariff surge has forced pharmaceutical companies to reevaluate sourcing strategies, often triggering shifts toward higher-cost suppliers or increased domestic manufacturing investments to maintain supply continuity.
More profound still is the impact of targeted tariffs on APIs sourced from China, which now carry duties exceeding 200%. Given that Chinese manufacturers supply nearly 40% of APIs for U.S. generic drugs, this levy has exacerbated cost volatility and threatened the availability of essential components used in cough hypersensitivity therapies. Experts warn that these barriers could prolong product launch timelines and escalate acquisition costs, undermining efforts to enhance patient access to novel antitussive agents.
Medical devices and diagnostic tools integral to CHS management-ranging from nebulizers to capsaicin challenge testing simulators-have also faced tariff-induced cost increases. With nearly 70% of U.S.–marketed medical devices manufactured abroad, the introduction of a 25% levy on supplies from Canada and Mexico has further strained procurement budgets. Healthcare providers are navigating these cumulative pressures by exploring alternative sourcing and collaborating with manufacturers to mitigate downstream price hikes, all while striving to preserve care quality and patient access.
Dissecting Multifaceted Patient, Provider, and Product Segmentation to Illuminate Opportunity Pathways in Cough Hypersensitivity Syndrome Therapies
The treatment landscape for CHS is shaped by multifaceted segmentation across care settings, reflecting varying patient needs and resource availability. In home care environments, patients often rely on self-administered antitussive therapies and nebulizer systems, whereas general hospitals prioritize rapid-acting inhalation treatments and emergency management protocols. Specialty hospitals, including ENT and pulmonology clinics, leverage advanced diagnostic platforms to tailor interventions, integrating both central neuromodulators and emerging peripheral antagonists based on clinical phenotyping.
Formulation preferences further delineate market opportunities and patient adherence profiles. While dry powder inhalers and metered dose inhalers offer precise, localized drug delivery for acute cough suppression, nebulizer solutions and oral syrups provide flexibility for at-home symptom control. Capsules and tablets, recognized for dosing convenience, remain popular for neuromodulatory agents, especially in patients requiring consistent systemic exposure throughout the day.
Prescription type and patient demographics intertwine to influence therapy adoption. Over-the-counter availability supports early symptom relief for adult populations, yet prescription-only access ensures medical oversight for geriatrics and pediatrics, where safety considerations demand careful titration. The adult cohort, representing the majority of diagnosed CHS cases, contrasts with the specialized needs of pediatric patients, whose neurodevelopmental considerations necessitate lower-dose formulations. Geriatric patients, prone to polypharmacy, underscore the importance of simplified dosing regimens and vigilant drug interaction management.
This comprehensive research report categorizes the Cough Hypersensitivity Syndrome Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Formulation
- Prescription Type
- Patient Age Group
- End User
Exploring Regional Dynamics Across the Americas, EMEA, and Asia-Pacific to Reveal Distinct Drivers Shaping Cough Hypersensitivity Syndrome Treatment Adoption
In the Americas, established healthcare infrastructures and robust reimbursement frameworks have accelerated the uptake of novel CHS therapies, particularly in the United States and Canada, where neuromodulators and P2X3 receptor antagonists have entered late-stage clinical evaluation. Clinicians benefit from broad access to multidisciplinary care teams, integrating pulmonology, otolaryngology, and speech therapy services to deliver comprehensive cough suppression programs and optimize patient outcomes.
Europe, the Middle East, and Africa (EMEA) present a mosaic of regulatory environments that influence market dynamics. Stringent approval pathways in the European Union have fostered cautious adoption of off-label neuromodulators, while emerging reimbursement models in select Middle Eastern countries have begun to recognize the long-term cost savings associated with effective CHS management. However, disparities in access remain pronounced, particularly in regions where specialty clinics and trained therapists are limited.
Across Asia-Pacific, rapid economic growth and expanding healthcare budgets are driving increased investment in chronic cough research and treatment availability. Clinical trial activity in countries such as Japan, Australia, and China has surged, with local subsidiaries of global pharmaceutical firms and regional biotech companies collaborating to evaluate P2X3 antagonists and behavioral interventions. These efforts highlight a dual focus on high-efficacy molecules and culturally adapted nonpharmacological programs, aiming to address rising patient demand and diverse environmental cough triggers across the region.
This comprehensive research report examines key regions that drive the evolution of the Cough Hypersensitivity Syndrome Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Market Participants and Emerging Biotech Innovators Pioneering Next-Generation Solutions for Chronic Cough Hypersensitivity Syndrome
Leading pharmaceutical companies have intensified R&D efforts to expand CHS treatment portfolios, leveraging both established drug classes and next-generation molecules. Merck & Co’s gefapixant, a pioneering P2X3 antagonist, stands at the forefront, having delivered consistent clinical efficacy in refractory chronic cough trials despite challenges related to taste disturbances. The molecule’s progression through phase III studies underscores the company’s commitment to addressing neural cough pathways at scale.
Biotech innovators have also emerged as critical market participants, advancing peripheral-targeted compounds with improved safety profiles. Bellus Health’s BLU-5937, characterized by high selectivity for the P2X3 receptor homotrimer and minimal taste-related side effects, is poised to reshape the treatment landscape upon successful clinical validation. Additional early-stage candidates, including S-600918 and BAY 1817080, reflect a growing emphasis on isoform-specific antagonism to optimize therapeutic windows and minimize central nervous system exposure.
Traditional neuromodulator manufacturers, such as Pfizer and Teva, continue to support off-label gabapentin and pregabalin use, while exploring innovative dosing regimens and fixed-dose combinations to enhance tolerability. Collaborations between pharmaceutical companies and specialized speech therapy networks further demonstrate the strategic shift toward integrated care models, where product development aligns with nonpharmacological expertise to deliver holistic solutions for CHS patients.
This comprehensive research report delivers an in-depth overview of the principal market players in the Cough Hypersensitivity Syndrome Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AstraZeneca PLC
- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche AG
- GlaxoSmithKline plc
- Haleon plc
- Johnson & Johnson
- Kyorin Pharmaceutical Co., Ltd.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Procter & Gamble Company
- Reckitt Benckiser Group plc
- Sanofi S.A.
- Shionogi & Co., Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- Vertex Pharmaceuticals Incorporated
- Viatris Inc.
- Zambon S.p.A.
Strategic Imperatives for Industry Leaders to Drive Innovation, Streamline Supply Chains, and Enhance Patient Access in Chronic Cough Therapies
To capitalize on the evolving CHS landscape, industry leaders should prioritize the development of highly selective P2X3 receptor antagonists that minimize off-target effects and facilitate regulatory approval. Strategic investment in dose-response research will be critical to balancing efficacy and tolerability, ensuring broad clinical adoption and positive payer coverage decisions.
Integrating nonpharmacological expertise into commercial strategies is equally vital. Establishing partnerships with speech-language pathology networks and training programs will expand access to behavioral cough suppression therapy, creating complementary revenue streams and reinforcing product value propositions. Such collaborations can also support robust real-world evidence generation, demonstrating long-term patient benefits and influencing guideline recommendations.
Mitigating supply chain risks stemming from escalating tariffs requires a diversified procurement approach. Companies should evaluate alternative API sourcing regions and consider localized manufacturing investments to reduce exposure to unilateral trade measures. Proactive engagement with government bodies and participation in industry consortia can further shape policy dialogues and safeguard critical supply lines for CHS treatment components.
Synthesizing Rigorous Qualitative and Quantitative Research Methodologies Underpinning Insightful Analysis of Cough Hypersensitivity Syndrome Treatment Trends
The insights presented herein derive from a rigorous research methodology combining extensive secondary literature review and primary qualitative engagements. Secondary data sources included peer-reviewed journals indexed in PubMed, clinical trial registries, and regulatory filings, with critical evaluation using standardized appraisal frameworks to ensure credibility and relevance. For instance, the systematic review and meta-analysis of neuromodulators informed comparative efficacy assessments and highlighted evidence gaps to guide future research priorities.
Primary research encompassed in-depth interviews with key opinion leaders in pulmonology, otolaryngology, and respiratory therapy, alongside structured consultations with pharmaceutical executives and healthcare policymakers. These dialogues provided nuanced perspectives on emerging treatment paradigms, reimbursement challenges, and regional access dynamics. Data triangulation techniques were employed to reconcile divergent viewpoints and validate thematic findings across sources.
Analytical processes integrated both qualitative coding of interview transcripts and quantitative synthesis of trial results, enabling identification of critical success factors and market barriers. Scenario modeling, informed by tariff projections and regulatory timelines, supported strategic forecasting while recognizing the limitations inherent in evolving clinical data. This structured approach underpins the robustness and actionable relevance of the conclusions drawn.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Cough Hypersensitivity Syndrome Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Cough Hypersensitivity Syndrome Treatment Market, by Formulation
- Cough Hypersensitivity Syndrome Treatment Market, by Prescription Type
- Cough Hypersensitivity Syndrome Treatment Market, by Patient Age Group
- Cough Hypersensitivity Syndrome Treatment Market, by End User
- Cough Hypersensitivity Syndrome Treatment Market, by Region
- Cough Hypersensitivity Syndrome Treatment Market, by Group
- Cough Hypersensitivity Syndrome Treatment Market, by Country
- United States Cough Hypersensitivity Syndrome Treatment Market
- China Cough Hypersensitivity Syndrome Treatment Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1431 ]
Converging Evidence on Therapeutic Advances and Unmet Needs Offering a Cohesive Outlook on the Future of Chronic Cough Management
The convergence of mechanistic pharmacotherapies and validated behavioral interventions establishes a multifaceted care model for CHS that addresses both neural sensitization and patient-centered management. Evidence supporting the efficacy of P2X3 receptor antagonists alongside speech therapy underscores the value of integrated treatment pathways tailored to individual phenotypes. This alignment of scientific innovation and clinical practice offers a cohesive framework for enhancing patient outcomes and reducing the burden of chronic cough.
Persistent barriers, including regulatory hurdles for novel compounds, supply chain uncertainties due to tariff expansions, and workforce limitations in specialized therapy delivery, highlight areas requiring targeted strategic initiatives. Addressing these challenges will demand coordinated efforts across industry stakeholders, healthcare systems, and policy makers to ensure that therapeutic advancements translate into accessible, cost-effective care for patients globally.
Looking ahead, continued investment in precision medicine, real-world evidence generation, and collaborative care models will be pivotal. By leveraging the insights and recommendations articulated in this report, organizations can position themselves at the vanguard of CHS management, driving innovation that meets evolving patient needs and shapes the future of chronic cough therapy.
Engage with Ketan Rohom to Secure Comprehensive Insights and Access Proprietary Market Research on Cough Hypersensitivity Syndrome Treatment
Elevate your strategic decision-making by securing our comprehensive market research insights on cough hypersensitivity syndrome treatment. Partner with Ketan Rohom, Associate Director of Sales & Marketing, to access proprietary analysis that delves into therapeutic innovations, competitive landscapes, and evolving regulatory environments. Connect today to ensure your organization capitalizes on emerging opportunities, addresses unmet patient needs, and stays ahead in this rapidly changing field. Engage now to transform insights into actionable strategies and unlock the full potential of your investment in chronic cough management.

- How big is the Cough Hypersensitivity Syndrome Treatment Market?
- What is the Cough Hypersensitivity Syndrome Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




