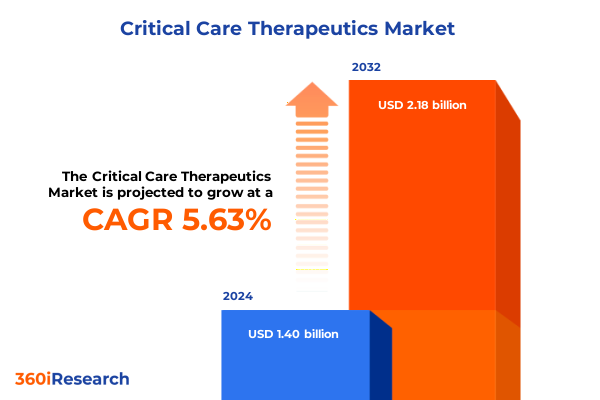
The Critical Care Therapeutics Market size was estimated at USD 1.48 billion in 2025 and expected to reach USD 1.57 billion in 2026, at a CAGR of 5.66% to reach USD 2.18 billion by 2032.
Introduction to the Critical Care Therapeutics Domain with Focus on Market Dynamics, Technological Advancements, and Evolving Clinical Innovation Paradigms
Critical care therapeutics encompass a wide array of interventions designed to sustain and restore physiological function in patients experiencing life-threatening conditions. This domain sits at the intersection of cutting-edge biomedical innovation and acute clinical practice, with a relentless focus on precision, safety, and patient outcomes. As the prevalence of complex, chronic diseases and acute emergencies continues to climb, the imperative to refine therapeutic modalities intensifies, prompting stakeholders to explore novel device architectures, pharmacological formulations, and integrated care pathways.
Within this dynamic environment, pharmaceutical companies, medical device manufacturers, and health systems must navigate stringent regulatory landscapes, cost containment pressures, and an accelerating pace of technological change. The synergy between digital health solutions-such as remote monitoring, data analytics, and artificial intelligence-and traditional critical care interventions has begun to redefine clinical workflows, creating new opportunities for efficiency and enhanced patient monitoring. Thus, understanding foundational market drivers, stakeholder incentives, and emerging innovation vectors is essential for decision-makers aiming to deliver life-saving therapies at scale while maintaining cost-effectiveness.
This introductory section lays the groundwork for a deep dive into the transforming forces shaping critical care therapeutics, setting the stage for strategic insights that will inform product development, policy engagement, and operational optimization.
Transformative Shifts Redefining Critical Care Therapeutics through Innovative Technologies, Policy Changes, and Evolving Patient Care Models
The critical care therapeutics landscape is undergoing profound transformation driven by breakthroughs in digital technology, shifting reimbursement models, and evolving patient expectations. Advancements in artificial intelligence have enabled predictive analytics to forecast patient deterioration hours before clinical signs emerge, thereby enabling early intervention and improved mortality outcomes. Simultaneously, the proliferation of tele-ICU platforms has extended specialized care capacity beyond urban academic centers, democratizing access to expert critical care oversight in rural and under-resourced settings.
On the policy front, a shift toward value-based care has altered the calculus for both providers and payers, incentivizing outcomes-focused models and bundled payment structures for intensive care episodes. This pivot has catalyzed collaborations between device manufacturers and health systems to co-develop solutions that align clinical efficacy with economic sustainability. Additionally, the integration of personalized medicine approaches-leveraging genomic data and biomarker-driven therapies-has started to redefine the therapeutic armamentarium for conditions such as sepsis, acute respiratory distress syndrome, and traumatic brain injury.
Taken together, these converging trends underscore the need for agility among market participants. Embracing interoperable platforms, forging strategic alliances, and investing in data-driven clinical decision support will be pivotal actions for organizations seeking to navigate this rapidly evolving terrain and maintain leadership in critical care innovation.
Examining the Cumulative Effects of Recent United States Tariffs on Critical Care Therapeutics Supply Chains, Cost Structures, and Market Access
United States tariffs introduced in early 2025 have exerted significant cumulative effects on the critical care therapeutics supply chain, reshaping cost structures and prompting a strategic reevaluation of sourcing strategies. Section 301 tariffs, initially imposed on certain medical device imports, were broadened midyear to include select intravenous infusion bags and catheters, triggering immediate cost escalations for hospital procurement teams. These levies have been further compounded by reciprocal trade measures from key export markets, accentuating pressure on multinational manufacturers to absorb or pass through additional duties.
In response, leading device producers have accelerated nearshoring initiatives, relocating critical component production to North American manufacturing hubs to mitigate tariff exposure while preserving quality and regulatory compliance. Concurrently, strategic inventory buffering and long-term supplier contracts have become commonplace, enabling organizations to hedge against future escalations and maintain uninterrupted product availability in high-acuity settings.
Moreover, the tariff environment has spurred renewed advocacy efforts by industry associations, engaging policymakers to refine exclusion processes for essential critical care supplies. These developments reinforce the necessity for proactive trade policy engagement and the adoption of agile procurement frameworks. Organizations that successfully integrate tariff impact modeling into their financial planning processes are better positioned to maintain margin integrity and uphold access to life-sustaining therapies across diverse patient populations.
Key Segmentation Insights Providing Deep Understanding of Product, Route of Administration, Patient Demographic, Application, and End-User Perspectives
A granular understanding of critical care market segmentation reveals nuanced insights into demand drivers and therapeutic priorities. Based on product type, the domain encompasses implantable devices, intravenous infusion bags, medications, monitoring devices, syringes and catheters, as well as ventilators, with medications further categorized into antibiotics, neuromuscular blockers, sedatives, and thrombolytics. Each product subset exhibits distinct innovation trajectories, regulatory considerations, and cost imperatives, shaping a mosaic of opportunity for specialized technology developers and pharmaceutical formulators.
Administration route segmentation underscores the relative prominence of inhalation, intravenous, and oral delivery mechanisms, each presenting unique pharmacokinetic profiles and patient compliance variables. Inhalation therapies, for example, have gained traction in acute respiratory management protocols, while intravenous formulations remain indispensable for rapid onset and precise dose titration in intensive care settings. Oral agents, though less common in the most acute phases, are critical for transition-of-care strategies and step-down protocols.
Patient demographic segmentation highlights the heterogeneity of critical care populations, spanning adult patients, geriatric cohorts, neonatal intensive care patients, and pediatric cases. The divergent physiological responses and comorbidity profiles across these groups necessitate tailored device ergonomics and dosage regimens, driving specialized R&D programs. Therapeutic application segmentation further delineates cardiovascular care, emergency resuscitation, neurological disorders, renal replacement therapy, and respiratory care, with subcategories such as intracranial pressure monitoring, traumatic brain injury, asthma, chronic obstructive pulmonary disease, and pulmonary edema. This layered segmentation enables stakeholders to align product pipelines with high-priority clinical niches and unmet medical needs.
End-user segmentation, encompassing ambulatory surgical centers, home healthcare, hospitals, and long-term care facilities, completes the landscape by illuminating distribution channels, reimbursement pathways, and service delivery models. Hospitals remain the epicenter of critical care deployment, yet the growing shift toward home-based interventions and outpatient centers is reshaping product design requirements and aftercare support services. Appreciating these segmentation dimensions is essential for market entrants and incumbents alike to craft targeted strategies that resonate with each stakeholder cohort.
This comprehensive research report categorizes the Critical Care Therapeutics market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Administration Routes
- Patient Demographics
- Therapeutic Application
- End-User
Regional Dynamics Shaping Critical Care Therapeutics Landscape with Insights into Americas, Europe, Middle East & Africa, and Asia-Pacific Trends and Drivers
Regional dynamics exert a profound influence on the evolution of critical care therapeutics, with the Americas, Europe, Middle East & Africa, and Asia-Pacific each presenting distinct regulatory environments, reimbursement infrastructures, and clinical imperatives. In the Americas, robust research and development ecosystems, coupled with a strong venture capital presence, accelerate the commercialization of disruptive technologies. North American health systems increasingly emphasize integrated care models and real-world evidence generation, fostering early adoption of digital health platforms and advanced monitoring solutions.
Meanwhile, the Europe, Middle East & Africa region grapples with heterogeneous reimbursement frameworks, ranging from single-payer systems in Western Europe to emerging private market dynamics in the Gulf Cooperation Council countries. This diversity necessitates nuanced market access strategies, including country-specific health technology assessments and payer engagement plans. Regulatory harmonization initiatives under the European Medical Device Regulation drive higher safety standards but also elongate time-to-market, compelling manufacturers to sequence launches strategically across key markets.
In Asia-Pacific, rapid population aging and growing healthcare investments in China, India, and Southeast Asian nations are fueling demand for cost-effective critical care interventions. Domestic manufacturing capabilities are expanding, supported by government incentives aimed at reducing import dependency. At the same time, partnerships between local firms and global incumbents are proliferating, enabling technology transfer and scaled production of essential devices. Across all regions, an overarching emphasis on value demonstration and lifecycle management is uniting stakeholders in a shared pursuit of sustainable, high-quality critical care delivery.
This comprehensive research report examines key regions that drive the evolution of the Critical Care Therapeutics market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Competitive Landscape Analysis Highlighting Leading Players, Strategic Partnerships, and Innovation Pipelines Driving Market Evolution
The competitive landscape in critical care therapeutics is defined by a mix of established multinationals, agile mid-tier innovators, and targeted startups. Global leaders have leveraged strategic partnerships to augment their portfolios, integrating digital health capabilities and cloud-based data analytics into legacy product lines. Mergers and acquisitions have been particularly prominent in the monitoring devices and ventilator segments, where scale economies and cross-selling opportunities drive consolidation.
This comprehensive research report delivers an in-depth overview of the principal market players in the Critical Care Therapeutics market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Abeona Therapeutics Inc.
- ADMA Biologics, Inc.
- Albumedix Ltd. by Sartorius AG
- Aspen Group
- Bayer AG
- Becton, Dickinson and Company
- Bio Products Laboratory Ltd.
- Collegium Pharmaceutical, Inc.
- CSL Limited
- Eli Lilly and Company
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Grifols SA
- Johnson & Johnson
- Kedrion Spa
- Merck & Co., Inc.
- Novartis AG
- Octapharma AG
- Pfizer Inc.
- Roche Holding AG
- Sanofi S.A.
- Shanghai RAAS
- Siemens Healthineers AG
- Takeda Pharmaceutical Co. Ltd.
Actionable Recommendations for Industry Leaders to Navigate Market Challenges, Capitalize on Opportunities, and Drive Sustainable Growth in Critical Care
Industry leaders must adopt a multifaceted approach to thrive amid intensifying competition and evolving external pressures. Supply chain diversification, including nearshore manufacturing and multi-supplier qualification, can mitigate tariff-related cost increases while enhancing resilience. Incorporating advanced tariff impact modeling into procurement analytics will aid in scenario planning and margin protection when trade policies shift.
Robust Research Methodology Underpinning Market Insights with Comprehensive Primary and Secondary Data Collection and Rigorous Analytical Framework
Our research methodology blends primary and secondary data sources to ensure robustness and depth. Extensive interviews with critical care physicians, hospital procurement managers, and regulatory experts provided firsthand insights into unmet needs, adoption barriers, and future technology desiderata. Complementing these qualitative inputs, secondary research encompassed comprehensive reviews of clinical trial registries, patent filings, regulatory databases, and peer-reviewed literature.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Critical Care Therapeutics market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Critical Care Therapeutics Market, by Product Type
- Critical Care Therapeutics Market, by Administration Routes
- Critical Care Therapeutics Market, by Patient Demographics
- Critical Care Therapeutics Market, by Therapeutic Application
- Critical Care Therapeutics Market, by End-User
- Critical Care Therapeutics Market, by Region
- Critical Care Therapeutics Market, by Group
- Critical Care Therapeutics Market, by Country
- United States Critical Care Therapeutics Market
- China Critical Care Therapeutics Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1431 ]
Concluding Insights Summarizing Strategic Imperatives and Key Takeaways to Guide Stakeholders in Critical Care Therapeutics Market
In synthesizing the critical care therapeutics landscape, several strategic imperatives emerge: prioritize innovation in high-acuity niches, strengthen supply chain adaptability to external shocks, and refine segmentation-based commercialization strategies. Stakeholders that align R&D investments with patient demographic and therapeutic application priorities will unlock enhanced clinical value and market differentiation.
Unlock Exclusive Critical Care Therapeutics Market Research Insights with Personalized Guidance from Ketan Rohom Associate Director Sales & Marketing
Seize the opportunity to elevate your strategic planning and operational excellence in critical care therapeutics by partnering directly with our Associate Director of Sales & Marketing, Ketan Rohom. With extensive expertise in life sciences and a deep understanding of the complexities that define critical care markets, Ketan is uniquely positioned to guide you through our comprehensive research findings and tailor insights to your organization’s specific needs.
Whether you are seeking to refine your product innovation roadmap, optimize supply chain resilience in the face of evolving tariff landscapes, or deepen your grasp of patient-centric segmentation, a one-on-one consultation will ensure you maximize the value of our market intelligence. Engage with Ketan to explore customized data visualizations, scenario planning tools, and strategic frameworks that align with your growth objectives.
Take decisive action today-connect with Ketan Rohom to secure your advanced copy of the report, unlock bespoke strategic recommendations, and drive sustainable competitive advantage in the critical care therapeutics arena.

- How big is the Critical Care Therapeutics Market?
- What is the Critical Care Therapeutics Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




