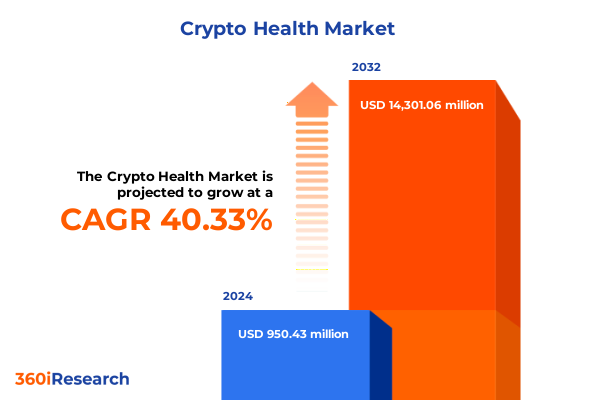
The Crypto Health Market size was estimated at USD 1.34 billion in 2025 and expected to reach USD 1.84 billion in 2026, at a CAGR of 40.21% to reach USD 14.30 billion by 2032.
Navigating the Intersection of Blockchain Innovation and Healthcare Delivery to Illuminate the Emergence of Crypto Health Ecosystems
The convergence of blockchain technology and healthcare is giving rise to Crypto Health as a distinct field, bringing unprecedented opportunities to enhance data integrity, patient engagement, and operational efficiency. Digital health records that were once siloed are now reimagined as immutable ledgers, granting stakeholders granular control over data provenance and access rights. This shift addresses longstanding concerns over security breaches and interoperability, while paving the way for novel token-based incentives that align provider and patient interests in a transparent ecosystem. Moreover, leading life sciences organizations are accelerating adoption of tokenization to link clinical trial data with real-world evidence, fostering richer datasets and improving long-term patient outcome analysis.
Transitioning from pilot projects to enterprise-grade deployments, healthcare CIOs are increasingly viewing blockchain infrastructure not as an experimental tool but as a foundational element for compliance automation and smart-contract adjudication. Immutable ledgers now serve as the backbone for regulatory compliance frameworks that adhere to HIPAA, GDPR, and emerging global data-sharing standards. As a result, the industry is witnessing an accelerated embrace of decentralized architectures across clinical data exchange, drug supply chain validation, and secure telemedicine services. This introduction sets the stage for exploring the transformative shifts reshaping the Crypto Health landscape.
Charting the Transformative Shifts That Are Redefining Data Integrity Patient Engagement and Tokenization in the Crypto Health Landscape
Over the past two years, the Crypto Health landscape has undergone transformative shifts that are redefining traditional healthcare paradigms. Decentralized clinical trials have emerged as a cornerstone application, leveraging blockchain’s immutable record-keeping to streamline patient recruitment, reduce fraud, and enhance transparency in data collection. Sponsors are now integrating tokenization protocols at the outset of trial design to link cohort data with diverse real-world evidence streams, thereby optimizing study efficiency and reducing duplicative data spend.
Simultaneously, the advent of BioNFTs has unlocked new funding models for R&D, enabling researchers to tokenize genetic data and intellectual property. These digital assets facilitate fractional ownership and unlock alternative capital sources for early-stage biotech ventures, fostering a more democratized investment landscape. Decentralized Autonomous Organizations (DAOs) tailored to healthcare governance are also gaining traction, empowering stakeholders to collectively manage resources, clinical protocols, and ethical frameworks without centralized intermediaries.
In parallel, innovative integrations of IoT devices with blockchain networks ensure end-to-end authenticity of sensor-generated health data, bolstering trust in remote monitoring and virtual consultations. These IoT solutions, coupled with emerging privacy-preserving cryptography, are setting new standards for patient data security while enabling seamless interoperability across disparate health systems. Together, these shifts illustrate how technological convergence is fostering a more resilient, patient-centric, and data-driven healthcare ecosystem.
Understanding the Multilayered Impact of 2025 US Trade Tariffs on Blockchain Enabled Medical Devices and Health Supply Chain Economics
The cumulative impact of United States tariff policy in 2025 is reshaping the supply chain economics for blockchain-enabled medical devices and health IT hardware. In January, the Office of the USTR finalized a four-year review under Section 301, increasing duties to 50 percent on imports of solar wafers and polysilicon and to 25 percent on select tungsten products, directly affecting semiconductor inputs essential to IoT devices and security modules used in clinical and telemedicine applications. These changes have driven up component costs, prompting manufacturers to reevaluate sourcing strategies and accelerate certification of domestic assembly lines.
Meanwhile, ongoing Section 232 investigations into pharmaceutical and semiconductor imports under the Trade Expansion Act highlight a broader intent to bolster domestic production, with proposed levies of 10–25 percent expected by mid-2025. Although certain medical equipment exclusions have been extended through August 2025, the specter of renewed duties has led to supply constraints and sporadic inventory shortages. In May, a 90-day reciprocal tariff reduction agreement temporarily reduced “reciprocal” duties from 125 percent to 10 percent on both sides, yet this relief excludes existing product-specific and Section 301 levies, perpetuating cost volatility.
Complementing these measures, a Presidential executive order has mandated the avoidance of tariff stacking when overlapping statutes apply to the same imported articles, aiming to curb excessive cumulative duty burdens. Collectively, these layered policies are compelling industry players to optimize manufacturing footprints, secure alternate supply routes, and incorporate tariff contingencies into strategic planning.
Uncovering Strategic Segmentation Drivers from Component Technologies to Token Archetypes Applications and End Users in the Crypto Health Market
Segmentation analysis reveals how distinct categories drive strategic insights and shape market dynamics in the Crypto Health sector. Component segmentation highlights that IoT devices and security modules within the hardware tier demand rigorous supply chain validation and robust compliance testing, while consulting, integration, and support services are critical for seamless deployment. Middleware platforms bridge on-premises and cloud-based infrastructures, and comprehensive solutions and platforms encompass everything from smart-contract orchestration to token management interfaces.
Token type segmentation underscores diverse utility models: governance tokens are facilitating decentralized decision-making on clinical consortium platforms; NFTs are revolutionizing patient credentialing and BioNFT intellectual property frameworks; security tokens underpin investment vehicles for healthtech startups; stablecoins are emerging as reliable payment rails for cross-border telemedicine services; and utility tokens incentivize data sharing by patients and providers.
Application segmentation drives innovation in clinical trials-where data management and patient recruiting tokens increase recruitment speed and data integrity-and in data security, where encryption algorithms and digital identity frameworks enhance patient privacy. In electronic health records, scalable storage and interoperability modules are enabling unified patient data ecosystems. Supply chain management utilizes inventory tracking and end-to-end traceability protocols, while telemedicine platforms integrate remote consultation and continuous vital-sign monitoring to expand care access.
End user segmentation shapes product roadmaps: clinics and hospitals prioritize operational efficiency, chronic care and home care segments emphasize continuous monitoring, government agencies and insurers focus on compliance and cost management, drug manufacturers and research organizations drive supply chain transparency and clinical validation, and both academic and private research institutes harness tokenized data for advanced analytics and translational studies.
This comprehensive research report categorizes the Crypto Health market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Component
- Token Type
- Application
- End User
Evaluating Regional Dynamics Shaping Adoption Patterns Across the Americas EMEA and Asia Pacific in the Emerging Crypto Health Industry
Regional dynamics play a pivotal role in shaping the adoption and regulatory frameworks of Crypto Health solutions. In the Americas, strong digital health infrastructure and well-established EHR standards have accelerated blockchain pilots, particularly in the United States, where healthcare providers and payers are integrating permissioned networks for secure data exchange. Government incentives and public-private partnerships have facilitated enterprise-grade deployments in telemedicine and supply chain management, while academic institutions lead in clinical trial tokenization research.
Europe, Middle East & Africa (EMEA) is characterized by stringent data privacy regulations such as GDPR, which have heightened the demand for blockchain-enabled interoperability solutions that ensure compliance and auditability. Cross-border healthcare initiatives within the EU are fostering consortium models, enabling shared governance frameworks and collective innovation hubs. In the Middle East, sovereign wealth funds are investing in blockchain-driven patient identity programs, and in Africa, decentralized telehealth platforms are widening access to care in underserved communities.
In Asia-Pacific, rapid digital transformation and supportive government policies have catalyzed blockchain adoption in healthcare supply chains, with major investments in smart contracts for pharmaceutical traceability and IoT-integrated remote monitoring networks. Countries such as Singapore and South Korea are advancing national health data tokenization pilots, while India’s healthcare startups are leveraging stablecoins to streamline micro-transactions in rural telemedicine services.
This comprehensive research report examines key regions that drive the evolution of the Crypto Health market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Key Innovators Driving Blockchain Healthcare Solutions from Global Giants to Specialized Crypto Health Startups Shaping the Future of Care
Leading technology firms and specialized startups are driving innovation in the Crypto Health ecosystem through strategic partnerships and product advancements. IBM’s blockchain platform has become a cornerstone for healthcare networks seeking interoperable and secure data frameworks, integrating smart-contract functionalities into claims adjudication and patient consent workflows. Microsoft’s Azure Blockchain Service empowers developers to build scalable solutions for clinical data exchange and supply chain validation, further solidifying its position in enterprise healthcare IT portfolios.
Change Healthcare, part of a major healthtech conglomerate, is leveraging blockchain to streamline revenue cycle management and claims processing, reducing administrative costs and improving data accuracy. Guardtime’s KSI blockchain underpins immutable audit trails for electronic health records and pharmaceutical logistics, while Hashed Health fosters consortium networking platforms that enhance stakeholder collaboration across care delivery ecosystems.
Innovative startups are also reshaping market boundaries. Medicalchain’s platform grants patients sovereign control over medical records, enabling secure data sharing with practitioners and researchers. Chronicled’s MediLedger network enhances drug traceability to combat counterfeiting and ensure regulatory compliance. Meanwhile, platforms like BurstIQ are scaling solutions for big-data management in healthcare, offering HIPAA-compliant tools for data monetization and licensing agreements.
This comprehensive research report delivers an in-depth overview of the principal market players in the Crypto Health market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Akiri Inc.
- Chronicled Inc.
- Doc.AI Inc.
- EncrypGen LLC
- Guardtime AS
- Hashed Health LLC
- Medicalchain SA
- Nebula Genomics Inc.
- Patientory Inc.
- ProCredEx Inc.
- SimplyVital Health Inc.
- Solve.Care Foundation
Developing Actionable Strategies for Industry Leaders to Accelerate Blockchain Integration Enhance Data Security and Optimize Token Economy Adoption in Healthcare
Industry leaders must adopt a multifaceted strategy to capitalize on emerging opportunities and navigate evolving challenges in Crypto Health. First, enterprises should establish cross-functional blockchain governance frameworks that integrate compliance, IT security, and clinical operations, ensuring alignment with HIPAA, GDPR, and new USTR directives on tariff stacking. By formalizing these structures, organizations can expedite approvals for pilot programs and achieve faster time-to-value.
Second, diversifying hardware sourcing is essential to mitigate the impact of Section 301 and Section 232 levies. Engaging with domestic semiconductor foundries, qualifying alternate IoT device suppliers, and pursuing tariff exclusion petitions will reduce cost volatility and safeguard supply chains. Concurrently, investing in green consensus protocols and energy-efficient IoT integrations will address sustainability mandates and lower operational overhead, as ecological manageability becomes a core market requirement.
Third, forging consortium alliances and public-private partnerships can accelerate interoperability standards and token-economy frameworks. Collaboration with regulatory bodies to develop standardized token definitions-governance, utility, and security-will enhance market confidence and streamline approval pathways. Finally, organizations should prioritize talent development in blockchain cybersecurity and smart-contract auditing, establishing centers of excellence to sustain innovation and ensure robust risk management across all Crypto Health initiatives.
Detailing the Rigorous Research Methodology Blending Primary Expert Insights with Secondary Data Sources to Illuminate the Crypto Health Market Landscape
This research employed a hybrid methodology combining primary and secondary data collection to deliver comprehensive insights into the Crypto Health market. Primary research involved in-depth interviews with more than twenty blockchain architects, healthcare CIOs, regulatory affairs specialists, and clinical trial directors. These discussions provided qualitative perspectives on implementation challenges, compliance considerations, and emerging token use-cases.
Secondary research comprised a thorough review of industry publications, peer-reviewed journals, corporate whitepapers, and government policy documents spanning USTR tariff notices, executive orders, and global health data regulations. Proprietary databases and trade associations supplied detailed information on technology partnerships, segmentation frameworks, and regional deployment case studies.
Data validation occurred through triangulation of differing viewpoints to reconcile discrepancies and ensure factual accuracy. A multi-stakeholder Delphi panel reviewed preliminary findings to refine strategic recommendations and confirm market dynamics. This rigorous approach guarantees that the executive summary reflects both real-world experiences and quantifiable policy impacts, offering a robust foundation for informed decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Crypto Health market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Crypto Health Market, by Component
- Crypto Health Market, by Token Type
- Crypto Health Market, by Application
- Crypto Health Market, by End User
- Crypto Health Market, by Region
- Crypto Health Market, by Group
- Crypto Health Market, by Country
- United States Crypto Health Market
- China Crypto Health Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 2862 ]
Synthesizing Key Takeaways to Illuminate the Strategic Imperatives and Growth Trajectories Shaping the Future of Crypto Health Ecosystems
The synthesis of market trends and policy shifts underscores critical imperatives for stakeholders in the Crypto Health ecosystem. Blockchain’s transition from experimental pilots to production deployments reaffirms its strategic role in securing health data, optimizing clinical operations, and enabling innovative financing models through tokenization. However, the specter of layered US tariffs and evolving regulatory requirements necessitates proactive supply chain and governance strategies.
Segmentation insights reveal that successful implementations hinge on tailored approaches across components, token types, applications, and end users, demanding nuanced value propositions and technical configurations. Regional nuances in infrastructure maturity, data privacy regulations, and public sector initiatives further underscore the need for agile, localized go-to-market frameworks. Meanwhile, partnerships between global technology leaders and specialized startups are catalyzing breakthroughs in interoperability and patient-centric service delivery.
Ultimately, organizations that adopt robust governance mechanisms, diversify sourcing, invest in talent, and engage in collaborative innovation will be best positioned to navigate emerging challenges and harness growth opportunities. These strategic imperatives will define the next phase of evolution for Crypto Health, driving improved patient outcomes, operational efficiencies, and sustainable ecosystem growth.
Partner with Ketan Rohom to Unlock Actionable Crypto Health Insights and Secure Your Comprehensive Executive Summary Market Research Report Today
Elevating your strategic positioning in the rapidly evolving Crypto Health domain begins with unlocking deep, data-driven insights tailored to your organizational goals. As an Associate Director specializing in sales and marketing for cutting-edge market research, Ketan Rohom offers a personalized consultation to align the executive summary’s findings with your most pressing challenges and opportunities. Whether you seek to refine your product roadmap, validate go-to-market strategies, or navigate complex regulatory and tariff landscapes, his expertise will ensure you derive maximum value from the full market research report.
Connect directly with Ketan to secure immediate access to proprietary case studies, detailed segmentation analysis, and in-depth regional breakdowns that will empower your leadership team to make informed decisions. This tailored engagement delivers actionable recommendations and competitive intelligence, enabling you to accelerate blockchain integration, optimize token economies, and fortify supply chain resilience.
Don’t let the dynamic shifts in the Crypto Health ecosystem outpace your organization. Reach out to Ketan Rohom today to purchase the comprehensive market research report and position your company at the forefront of innovation. Empower your strategic planning with a data-rich roadmap crafted by industry experts who understand the intersection of finance, technology, and healthcare.

- How big is the Crypto Health Market?
- What is the Crypto Health Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




