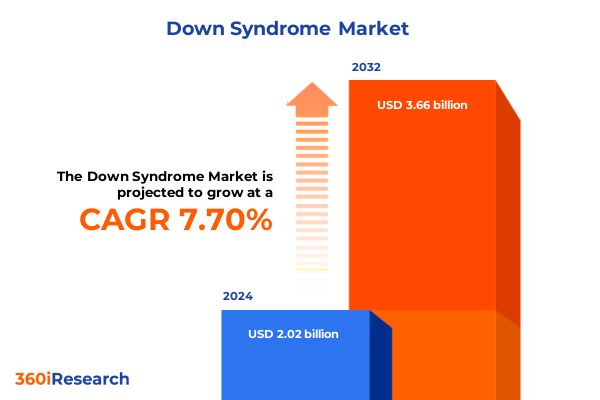
The Down Syndrome Market size was estimated at USD 2.18 billion in 2025 and expected to reach USD 2.33 billion in 2026, at a CAGR of 7.67% to reach USD 3.66 billion by 2032.
Catalyzing Inclusive Progress Through Transformative, Comprehensive Strategies in Down Syndrome Diagnostics, Care, and Collaboration
The landscape of Down Syndrome diagnostics and care is evolving at an unprecedented pace, requiring a nuanced understanding of both clinical and commercial dimensions. This introduction sets the foundation by highlighting the urgency of advancing diagnostic capabilities and therapeutic interventions to improve outcomes for individuals with Down Syndrome. At its core, this executive summary synthesizes the key drivers shaping the market, from technological breakthroughs in genetic screening to policy developments that influence access and reimbursement.
Amid growing societal emphasis on inclusion and personalized healthcare, stakeholders across the continuum-clinicians, payers, policymakers, and patient advocates-are aligning around shared objectives. These objectives include early, accurate detection of trisomy 21, expansion of supportive services, and development of targeted therapies. By framing the current environment through the lenses of innovation, regulatory action, and stakeholder collaboration, this opening section establishes a shared context from which the subsequent analyses emerge.
Unveiling Revolutionary Advances That Are Reshaping Down Syndrome Screening, Treatment Pathways, and Stakeholder Engagement Across the Ecosystem
In recent years, the Down Syndrome ecosystem has experienced a wave of transformative innovations that are redefining screening protocols and therapeutic approaches. One foundational shift has been the widespread adoption of noninvasive prenatal screening techniques, which enable earlier and safer detection of trisomy 21 without the miscarriage risk associated with invasive procedures. Complementing this, advanced biochemical assays and high-throughput genetic panels are providing clinicians with more precise diagnostic data, facilitating personalized care plans.
Simultaneously, the research community has made significant strides in emerging gene editing platforms, including CRISPR-based preclinical studies aimed at addressing core genetic mechanisms. Parallel progress in pharmacological therapies-spanning antiepileptic and behavioral medications-underscores a holistic approach to symptom management and quality-of-life enhancement. Beyond diagnostics and drugs, the integration of digital health tools and remote monitoring capabilities is empowering families and care teams to collaborate more effectively, while proactive policy efforts are driving broader insurance reimbursement for early intervention and genetic counseling services.
As a result of these converging trends, the Down Syndrome care continuum is becoming more interconnected and data-driven, enabling faster adoption of best practices and more dynamic feedback loops between patients, providers, and payers.
Analyzing the Comprehensive Effects of 2025 United States Tariff Policies on Down Syndrome Diagnostics and Care Ecosystem
The implementation of new United States tariffs in 2025 is exerting a multifaceted influence on the Down Syndrome diagnostics and care landscape, particularly within the realm of imported laboratory reagents and specialized equipment. For instance, the 10% universal tariff on most imported goods, combined with country-specific duties of up to 145% on Chinese-origin lab supplies, has driven up costs for critical reagents used in biochemical assays and genetic panel testing. These increased expenses are being felt by diagnostic centers that rely on fluorescence in situ hybridization and polymerase chain reaction kits sourced internationally ﹘ challenges that have prompted several service providers to explore local sourcing alternatives or negotiate supply contracts to mitigate financial strain.
Moreover, high tariffs on medical devices from Canada and Mexico-regional partners historically exempt from broad-duty measures-have disrupted procurement timelines for equipment used in noninvasive prenatal testing and invasive diagnostic procedures. Hospital administrators report extended lead times for key instruments, such as amniocentesis monitors and sequencing platforms, with some facilities deferring routine upgrades to manage budget pressures. In parallel, major manufacturers have begun shifting toward domestic assembly and localized supply chains. GE Healthcare, for example, anticipates a $500 million tariff-related impact in 2025 but expects mitigation strategies to reduce this burden in subsequent years.
These tariff-driven dynamics are creating ripple effects across patient access and service delivery. Diagnostic centers are passing a portion of increased costs to insurers and patients, which could influence demand patterns for prenatal screening services and postnatal genetic counseling. At the same time, policy advocacy groups are intensifying efforts to secure exemptions for life-saving diagnostic goods, underscoring the tension between trade policy objectives and public health imperatives.
Unlocking Market Complexity Through In-Depth Segmentation of Down Syndrome Screening, Treatment, Service, User, and Distribution Channels
The Down Syndrome market is characterized by a rich tapestry of testing methodologies, each unlocking unique insights at different stages of life. Newborn screening protocols leverage both biochemical assays and comprehensive genetic panels to identify trisomy 21 shortly after birth, enabling timely interventions. Meanwhile, postnatal diagnoses rely on chromosomal karyotyping, fluorescence in situ hybridization, and polymerase chain reaction methods to confirm clinical suspicions and guide family counseling. Prenatal assessments offer both invasive diagnostic testing and noninvasive prenatal testing, catering to risk profiles and patient preferences with a balance of accuracy and safety.
Treatment paradigms are equally diverse, encompassing gene therapies that harness CRISPR-based or viral vector-based delivery systems to explore potential disease-modifying pathways, pharmacological regimens ranging from antiepileptic drugs to behavioral medications, and supportive therapies delivered through occupational, physical, and speech modalities. These treatment streams address not only the core genetic etiology but also the associated developmental and neurological challenges that manifest throughout the individual’s lifespan.
Service offerings extend from care management solutions-spanning care coordination to home nursing-to early intervention initiatives that integrate educational and physical therapy programs, and genetic counseling services available in both postnatal and prenatal contexts. End users range broadly, including diagnostic centers, hospitals, home care providers, and research institutes committed to advancing knowledge and practice. Finally, distribution channels vary from direct sales and hospital pharmacies to online platforms and retail pharmacies, each channel playing a distinct role in ensuring that diagnostic tools and therapeutics reach the patients who need them.
This comprehensive research report categorizes the Down Syndrome market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Test Type
- Treatment Type
- Service Type
- End User
- Distribution Channel
Illuminating Regional Dynamics and Best Practice Variations in Down Syndrome Care Across the Americas, Europe, Middle East & Africa, and Asia-Pacific
Across the Americas, robust public health infrastructures and growing advocacy movements are accelerating the adoption of advanced Down Syndrome screening and care protocols. Government initiatives aimed at expanding newborn screening panels and enhancing insurance coverage for noninvasive prenatal tests have fostered early detection and intervention programs. These developments are complemented by partnerships between academic medical centers and community organizations that drive awareness and support for families.
In Europe, the Middle East, and Africa, heterogeneous healthcare systems present both challenges and opportunities. While several Western European nations have integrated comprehensive genetic counseling and reimbursement frameworks, emerging markets within EMEA are focused on building laboratory capacity and developing localized manufacturing to reduce dependency on imports. Policy collaboration among regional consortia is strengthening regulatory pathways for novel therapies and diagnostic assays, although access disparities remain pronounced in underserved areas.
The Asia-Pacific region is witnessing rapid growth in diagnostic and therapeutics uptake, driven by private sector investments and public–private partnerships. Countries such as Japan and Australia are leading in regulatory approvals for innovative genetic tests, whereas developing APAC markets are prioritizing scalable infrastructure for high-throughput screening. Cross-border collaborations and technology transfer agreements are deepening the regional ecosystem, positioning Asia-Pacific as a pivotal hub for both clinical research and commercial expansion in Down Syndrome care.
This comprehensive research report examines key regions that drive the evolution of the Down Syndrome market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Pioneers and Innovators Driving Progress in Down Syndrome Screening, Therapeutics, and Support Services Worldwide
Major multinational diagnostics companies are shaping the trajectory of Down Syndrome screening with a steady stream of assay enhancements and expanded test menus. Industry leaders in genetic sequencing and array technologies are collaborating with clinical laboratories to validate next-generation panels that improve detection accuracy. Simultaneously, pharmaceutical firms specializing in neurology and rare disorders are advancing small molecule and biologic candidates targeting cognitive and developmental outcomes associated with trisomy 21.
Gene therapy innovators are forging alliances with academic research centers, leveraging CRISPR-based platforms and viral vectors to explore potential corrective approaches at the chromosomal level. In the realm of supportive care, specialized service providers are scaling up personalized therapy programs that integrate digital monitoring tools to measure progress and adjust interventions in real time.
Within this dynamic landscape, a growing number of biotech start-ups are emerging with differentiated diagnostic platforms, while established medical device manufacturers are investing in localized production to mitigate trade-related disruptions. Venture capital flows are increasingly directed toward companies demonstrating strong evidence of clinical utility and scalable delivery models, underscoring the competitive imperative to balance innovation with affordability.
This comprehensive research report delivers an in-depth overview of the principal market players in the Down Syndrome market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AC Immune SA
- Aelis Farma
- Alzheon Inc
- Annovis Bio Inc
- Aprhica Therapeutics
- AstraZeneca Plc
- Bayer AG
- Biogen Inc
- Bristol-Myers Squibb Company
- Connecta Therapeutics
- Eisai Co Ltd
- Eli Lilly and Company
- F Hoffmann-La Roche Ltd
- Ionis Pharmaceuticals Inc
- Johnson & Johnson Services Inc
- Karyopharm Therapeutics
- Merck & Co Inc
- NeuroNascent Inc
- Novartis AG
- OPKO Health Inc
- Perha Pharmaceuticals
- Pfizer Inc
- Sanofi SA
- Takeda Pharmaceutical Company Limited
Strategic Actions to Empower Industry Leaders and Foster Sustainable Growth in Down Syndrome Diagnostics and Therapy Markets
Industry leaders must prioritize strategic investments in emerging diagnostic and therapeutic technologies that have demonstrated clinical promise. By forging early partnerships with academic research networks, organizations can accelerate validation studies and shorten time to market. It is equally essential for executives to engage proactively with regulatory bodies and advocacy groups, ensuring that policy frameworks support rapid adoption of noninvasive screening methods and expanded access to genetic counseling services.
Operational resiliency should be reinforced through diversified sourcing strategies that balance domestic production with selective international procurement, thereby reducing vulnerability to trade disruptions. To enhance patient engagement and adherence, companies are advised to co-develop digital platforms with caregivers and clinician end users, enabling real-time monitoring of therapy outcomes and facilitating seamless data sharing across care teams.
Finally, stakeholders are encouraged to invest in outcomes research that quantifies long-term benefits of early intervention programs, generating evidence to inform reimbursement negotiations and payer relationships. By integrating these strategic actions, leaders can not only navigate the evolving market landscape but also drive meaningful improvements in the lives of individuals with Down Syndrome.
Methodological Framework and Rigorous Approaches Underpinning the Down Syndrome Market Analysis and Insight Generation
This research combines rigorous secondary source analysis with primary data collection and expert consultation to ensure robust insights. Peer-reviewed publications, regulatory filings, and industry white papers were systematically reviewed to map the latest advancements in diagnostic modalities, therapeutic development, and service delivery models. Government and payer databases provided context on policy trends and reimbursement landscapes.
Primary research involved semi-structured interviews with healthcare professionals, laboratory directors, policy experts, and patient advocacy leaders to validate findings and uncover real-world operational challenges. Data triangulation techniques were employed to reconcile quantitative metrics and qualitative feedback, enhancing confidence in key conclusions.
Analytical frameworks, including SWOT analysis and Porter’s Five Forces, were applied to evaluate competitive dynamics, while scenario modeling assessed the potential impacts of trade policies and regulatory shifts. Segmentation analyses were executed to dissect market opportunities across test types, treatment modalities, service offerings, end users, and distribution channels, ensuring nuanced strategic recommendations.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Down Syndrome market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Down Syndrome Market, by Test Type
- Down Syndrome Market, by Treatment Type
- Down Syndrome Market, by Service Type
- Down Syndrome Market, by End User
- Down Syndrome Market, by Distribution Channel
- Down Syndrome Market, by Region
- Down Syndrome Market, by Group
- Down Syndrome Market, by Country
- United States Down Syndrome Market
- China Down Syndrome Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 2385 ]
Synthesizing Insights and Charting the Next Steps for Advancing Down Syndrome Care Through Multi-Stakeholder Collaboration
This executive summary has illuminated the critical intersections between technological innovation, policy evolution, and market dynamics shaping Down Syndrome diagnostics and care. From the growing sophistication of noninvasive prenatal screening to the strategic responses prompted by trade-related pressures, the analysis underscores the importance of an integrated approach. Stakeholders equipped with these insights can better navigate complexities, optimize resource allocation, and align with patient-centric outcomes.
Moving forward, collaboration among diagnostic developers, therapy innovators, healthcare providers, and advocacy groups will be paramount to sustaining momentum. By leveraging data-driven strategies and fostering transparent dialogue with policymakers, the community can advance access, affordability, and quality of care for individuals with Down Syndrome worldwide.
As the market continues to mature, the agility of industry participants in adapting to emerging trends will determine their ability to drive both scientific progress and social impact. This synthesis serves as a blueprint for informed decision-making and collective action in support of a more equitable and effective care ecosystem.
Engage with Ketan Rohom to Secure In-Depth Market Intelligence and Drive Innovation in Down Syndrome Research and Commercial Strategies
To explore this comprehensive analysis in greater depth and to tailor strategic initiatives for your organization, reach out to Ketan Rohom, Associate Director of Sales & Marketing at 360iResearch. Engaging with Ketan offers direct access to the full scope of proprietary insights, including detailed evaluations of diagnostic innovations, therapy pipelines, regional dynamics, and competitive intelligence. His expertise in guiding decision-makers through complex market landscapes ensures that you receive actionable intelligence aligned with your organizational priorities. Whether you aim to refine your product development roadmap, optimize market entry plans, or strengthen partnerships with advocacy groups and policymakers, securing this report will equip you with the foresight needed to stay ahead. Connect with Ketan Rohom to obtain your copy of the detailed Down Syndrome Market Research Report and begin leveraging data-driven strategies that will drive innovation and growth in your organization.

- How big is the Down Syndrome Market?
- What is the Down Syndrome Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




