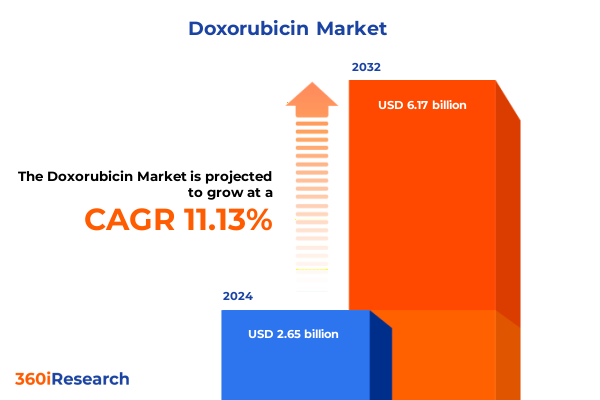
The Doxorubicin Market size was estimated at USD 2.95 billion in 2025 and expected to reach USD 3.28 billion in 2026, at a CAGR of 11.13% to reach USD 6.17 billion by 2032.
Revealing the Multifaceted Role of Doxorubicin as a Cornerstone Anthracycline Therapy Driving Modern Oncology Practices
Doxorubicin, an anthracycline antibiotic first isolated from Streptomyces peucetius var. caesius, remains a cornerstone in chemotherapy protocols due to its potent antineoplastic activity and proven clinical efficacy. Its cytotoxic mechanism involves intercalation between DNA base pairs and inhibition of topoisomerase II, leading to disrupted DNA replication and the formation of oxygen free radicals that contribute to both its therapeutic and cardiotoxic effects. Since its initial U.S. approval in 1974, doxorubicin has been supplied in multiple formulations, including intravenous injections and liposomal carriers, with evolving pharmacokinetic profiles shaping its therapeutic window.
Clinically, doxorubicin is approved for a broad spectrum of cancers, spanning acute leukemias, lymphomas, breast and ovarian carcinomas, soft tissue sarcomas, and pediatric solid tumors. While its high potency underscores its value, the drug’s use is tempered by cumulative dose–related cardiomyopathy, myelosuppression, and extravasation risks, necessitating vigilant monitoring throughout treatment cycles. Ongoing efforts to mitigate adverse effects have spurred developments in cardioprotective agents and advanced delivery systems, reflecting the continuous drive to enhance the therapeutic index of this essential chemotherapeutic agent.
Innovations in Liposomal and Stimuli Responsive Delivery Systems Are Revolutionizing the Therapeutic Impact of Doxorubicin in Cancer Care
Innovations in drug delivery have transformed the utilization of doxorubicin beyond its conventional free form by integrating nanotechnology and stimuli-responsive carriers that selectively release payloads at tumor sites. Thermosensitive liposomes, activated through focused ultrasound or hyperthermia, facilitate localized doxorubicin release, achieving up to a fifteen-fold increase in tissue concentration compared with non-heated controls in preclinical models. Further optimization of lyso-thermosensitive liposomes by adjusting phospholipid transition temperatures has enhanced stability and cellular uptake under ultrasonic hyperthermia, significantly improving antitumor efficacy in murine colon carcinoma models.
Beyond thermal triggers, magnetic thermosensitive liposomes have been engineered to integrate diagnostic imaging with targeted therapy. Chelated gadolinium imparts magnetic guidance and hyperthermia control under ultra-high-field MRI, enabling temperature-controlled doxorubicin release while concurrently enhancing contrast for in vivo monitoring. In parallel, clinical trials have demonstrated the feasibility of extracorporeal MR-guided high intensity focused ultrasound to induce mild hyperthermia and trigger drug delivery, revealing the potential to achieve chemo-ablative responses in refractory liver tumors without invasive ablation techniques.
Assessing the Compound Effects of 2025 U.S. Tariffs on Pharmaceutical Supply Chains and Doxorubicin Manufacturing Costs
In 2025, the U.S. enacted a sweeping 10% global tariff on all imports, encompassing active pharmaceutical ingredients (APIs) and finished oncology drugs, while imposing tariffs up to 245% on APIs sourced from China and 20-25% on imports from India, sharply elevating production costs for generic and branded drug manufacturers alike. Concurrently, a proposed 25% tariff on finished pharmaceutical goods threatens to pass on cost increases to patients, potentially raising U.S. drug prices by up to 12.9% if duties are fully absorbed through the supply chain.
These cumulative trade measures exacerbate existing vulnerabilities in doxorubicin supply chains, which are heavily dependent on overseas API production. Europe supplies a significant share of high-value biologics and specialized intermediates, while China and India contribute over 70% of generic APIs used in U.S. drug manufacturing. The sudden imposition of tariffs introduces delays at customs, risk of shortages for critical oncology treatments, and increased lead times for manufacturers adapting to customs barriers. In the short to medium term, chemotherapy infusion centers and hospital pharmacies may experience price volatility and intermittent drug availability, underscoring the urgency of supply chain diversification and onshore production initiatives to maintain treatment continuity.
Decoding Patient Populations and Route Variations to Uncover Strategic Segments Shaping the Doxorubicin Market Landscape
A nuanced segmentation of the doxorubicin market reveals distinct patient cohorts and product formats driving clinical adoption and resource allocation. Within breast cancer applications, first-line regimens remain foundational, but the transition to second and third-line therapies underscores evolving resistance patterns. Kaposi’s sarcoma, leukemia, and ovarian cancer segments exhibit unique treatment protocols and dosing paradigms, informing tailored access strategies at referral centers and specialty clinics. Concurrently, the availability of lyophilized powder formulations caters to flexible pharmacy compounding practices, while non-pegylated and pegylated liposomal solutions enable extended circulation and reduced cardiotoxicity profiles, shaping procurement and reimbursement negotiations across healthcare systems.
Distribution channels and end-user settings further delineate market dynamics. Hospital pharmacies and ambulatory surgical centers maintain direct infusion hubs, whereas online and retail pharmacies expand patient-administered therapies for select indications. Cancer treatment centers integrate specialized infusion protocols, while home care settings leverage portable infusion devices. Venous access routes influence clinical workflow, with central venous catheters preferred for continuous or high-concentration infusions to mitigate extravasation risk, and peripheral lines reserved for intermittent dosing in robust patients. Finally, adult and pediatric patient groups demand differentiated dosing regimens, safety monitoring, and formulation handling, emphasizing the need for age-specific clinical pathways and caregiver support services.
This comprehensive research report categorizes the Doxorubicin market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Indication
- Formulation
- Administration Route
- Patient Age Group
- Distribution Channel
- End User
Exploring Regional Divergences in Doxorubicin Adoption Patterns Across Americas, Europe Middle East & Africa, and Asia-Pacific Healthcare Systems
Regional variations in healthcare infrastructure, reimbursement policies, and epidemiological trends shape the demand and delivery of doxorubicin therapies across the Americas, Europe Middle East & Africa, and Asia-Pacific. In the United States, breast cancer remains the most common malignancy among women after skin cancer, accounting for roughly 30% of new female cancer diagnoses in 2025. Despite declining mortality rates driven by early detection and targeted treatments, incidence among women under 50 continues to rise by approximately 1.4% annually, prompting expanded screening guidelines and risk-reduction initiatives.
Within Europe, Middle East, and Africa, high-income nations in Western and Eastern Europe have experienced modest decreases in age-standardized breast cancer incidence, even as North Africa and the Middle East face growing case burdens due to demographic shifts and limited screening penetration. In Asia-Pacific, population growth and increasing human development indices correlate with rising incidence and mortality rates; in 2022 alone, Asia accounted for approximately 42.9% of global breast cancer cases, with mortality disproportionately high in low-HDI regions where delayed diagnosis and treatment access barriers persist. These regional insights emphasize the need for culturally tailored awareness campaigns, funding for diagnostic infrastructure, and public-private partnerships to expand equitable access to quality oncology care.
This comprehensive research report examines key regions that drive the evolution of the Doxorubicin market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Stakeholders and Their Strategic Initiatives Impacting Doxorubicin Production Delivery and Market Competition Dynamics
Key stakeholders in the doxorubicin landscape encompass established pharmaceutical giants, generics manufacturers, and nimble specialty producers. Pfizer’s Adriamycin, first approved in 1974, continues to leverage a robust global distribution network and stringent quality controls, reinforcing its presence in high-volume oncology centers and academic hospitals. Johnson & Johnson’s Doxil, a pegylated liposomal doxorubicin formulation, exemplifies premium carrier technology with enhanced pharmacokinetics, while generic entrants such as Teva and Sandoz capitalize on economies of scale to supply lyophilized injections and standard liposomal solutions across multiple markets.
Recent AB-rated generic launches by Padagis, in partnership with Ayana Pharma, have introduced competitive liposomal alternatives, diversifying hospital formularies and driving price negotiations in mature markets. Beyond conventional producers, biotechnology startups and research institutions are pursuing cardioprotective co-therapy innovations using dexrazoxane and exploring device-based delivery systems to minimize off-target effects. Early-stage ventures integrating MR-guided high intensity focused ultrasound with thermosensitive liposomes signal emerging white-space opportunities, setting the stage for next-generation platform collaborations aimed at redefining therapeutic indices in solid tumors.
This comprehensive research report delivers an in-depth overview of the principal market players in the Doxorubicin market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Apotex Inc.
- Baxter International Inc.
- Cadila Pharmaceuticals Ltd.
- Cipla Limited
- Dr. Reddy's Laboratories Limited
- Fresenius Kabi AG
- Getwell Oncology Pvt Ltd.
- Glenmark Pharmaceuticals Ltd.
- Hikma Pharmaceuticals PLC
- Janssen Global Services, LLC By Johnson & Johnson Services, Inc.
- LGM Pharma, LLC
- Manus Aktteva Biopharma LLP
- Meiji Holdings Co., Ltd.
- Merrimack Pharmaceuticals, Inc.
- Novartis AG
- Pfizer Inc.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- TTY Biopharma Company Limited
Crafting Strategic Imperatives for Industry Leaders to Navigate Innovation Supply Chain Challenges and Regulatory Landscapes in Doxorubicin Development
To navigate evolving trade policies and supply chain disruptions, industry leaders should prioritize diversified sourcing strategies by establishing alternative API supply agreements in regions exempt from high tariffs, including select European and North American manufacturers. Investing in domestic production facilities for both active ingredients and final dosage forms will mitigate reliance on volatile import channels and strengthen resilience against future trade fluctuations. Concurrently, forging strategic alliances with logistics providers and specialty distributors can streamline customs processes and ensure prioritized clearance for oncology products subject to elevated duties.
On the innovation front, stakeholders should accelerate partnerships with academic centers to co-develop targeted delivery platforms, leveraging thermosensitive and magnetic liposomal carriers validated in early clinical trials. Collaborative programs with medical device firms specializing in focused ultrasound and hyperthermia technologies can unlock new clinical value propositions and extend patent life through device-drug combination patents. Finally, engaging proactively with regulatory agencies to secure and sustain temporary tariff exemptions for critical healthcare supplies, supported by real-world impact data on patient access and outcomes, will preserve treatment affordability and maintain uninterrupted patient care cycles.
Elucidating Rigorous Research Frameworks and Analytical Techniques Employed to Unveil Comprehensive Insights into the Doxorubicin Ecosystem
This analysis was underpinned by a rigorous research methodology combining primary data from regulatory filings, customs tariff records, and expert interviews with oncologists, pharmacists, and supply chain executives. Secondary research encompassed peer-reviewed journals, clinical trial registries, and government databases such as FDA’s DailyMed prescribing information to verify formulation specifics and administration protocols. Trade policy insights were sourced from authoritative finance and policy publications, including CNBC reports on tariff impacts and Reuters commentary on reshoring investment trends.
The segmentation framework integrated industry taxonomies for indication, formulation, distribution channels, end-user settings, route of administration, and patient demographics, synthesized through a cross-validation process using academic literature and health technology assessment guidelines. Regional analyses leveraged GLOBOCAN and American Cancer Society statistics to capture epidemiological divergences, while company profiles were constructed from U.S. FDA approvals, press releases, and proprietary patent databases. Data triangulation and stakeholder validation workshops ensured consistency and actionable relevance, reinforcing the robustness of strategic recommendations presented herein.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Doxorubicin market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Doxorubicin Market, by Indication
- Doxorubicin Market, by Formulation
- Doxorubicin Market, by Administration Route
- Doxorubicin Market, by Patient Age Group
- Doxorubicin Market, by Distribution Channel
- Doxorubicin Market, by End User
- Doxorubicin Market, by Region
- Doxorubicin Market, by Group
- Doxorubicin Market, by Country
- United States Doxorubicin Market
- China Doxorubicin Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1431 ]
Synthesizing Critical Learnings and Future Prospects to Chart the Continued Evolution of Doxorubicin’s Role in Oncological Therapies
This executive summary has highlighted the enduring clinical significance of doxorubicin and the transformative potential of advanced delivery technologies to enhance therapeutic outcomes. The integration of thermosensitive liposomes, magnetic guidance, and focused ultrasound heralds a new era in precision oncology, bridging decades-old efficacy with cutting-edge innovation. Concurrently, the cumulative impact of U.S. tariffs underscores the critical importance of supply chain diversification and policy engagement to safeguard patient access and cost stability across global markets.
Segmentation analysis revealed distinct clinical niches and operational considerations, from line-specific indication protocols to distribution channel optimization and age-tailored dosing strategies. Regional insights emphasized the heterogeneous nature of disease burden and healthcare infrastructure across the Americas, Europe Middle East & Africa, and Asia-Pacific, necessitating tailored market strategies and cross-border collaborations. Leading companies and emerging entrants alike must pursue strategic investments in onshore manufacturing, real-world evidence generation, and device-drug combination partnerships to maintain competitive advantage and drive sustainable growth in the dynamic doxorubicin landscape.
Connect with Ketan Rohom for Exclusive Insights and Access to Comprehensive Doxorubicin Market Research
To explore a comprehensive, data-driven analysis covering regulatory impacts, segmentation dynamics, and competitive landscapes in the global doxorubicin market, we invite you to engage directly with Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch. By connecting with Ketan, you will gain exclusive access to in-depth research findings, tailored strategic recommendations, and ongoing market intelligence updates designed to support informed decision-making in your organization. Reach out to Ketan to secure your copy of the full market research report and position your company at the forefront of innovation, policy adaptation, and supply chain optimization in the evolving oncology therapeutics sector.

- How big is the Doxorubicin Market?
- What is the Doxorubicin Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




