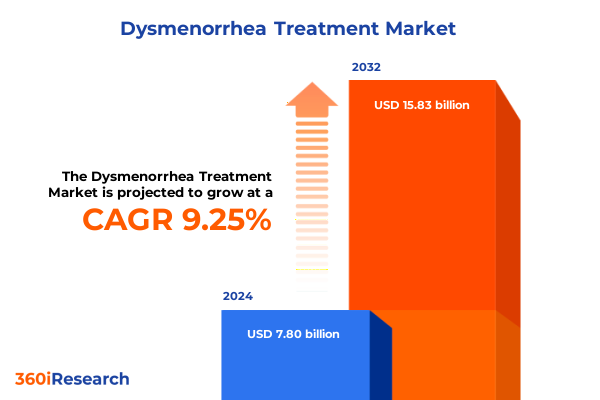
The Dysmenorrhea Treatment Market size was estimated at USD 8.51 billion in 2025 and expected to reach USD 9.24 billion in 2026, at a CAGR of 9.28% to reach USD 15.83 billion by 2032.
Understanding the Urgency of Addressing Dysmenorrhea: Context, Challenges, and Strategic Imperatives for Stakeholders in Women's Health Markets
Dysmenorrhea, characterized by cramping pain during menstruation, affects a substantial proportion of menstruating individuals globally, with the United States experiencing high prevalence rates that underscore its significance as a public health concern. The condition often manifests as both primary and secondary forms, with varying etiologies that challenge uniform treatment strategies. Pain intensity and associated symptoms such as nausea, headache, and fatigue contribute to a substantial burden on daily functioning and quality of life.
Chronic discomfort and pain lead to missed workdays, decreased productivity, and increased healthcare utilization, drawing attention from payers, providers, and consumer wellness platforms alike. Recent patient surveys indicate growing demand for integrated care pathways that blend clinical interventions with complementary therapies, reflecting a broader shift toward holistic health management. Furthermore, heightened awareness of non-pharmacological options and personalized medicine frameworks has created fertile ground for innovation among life sciences, technology, and wellness stakeholders.
In response to these evolving dynamics, this executive summary distills critical insights into market shifts, policy influences, and segmentation strategies that will shape the future of dysmenorrhea management. It outlines the transformative impacts of emerging modalities, assesses regulatory and tariff considerations, and identifies key patient, product, and regional dimensions driving strategic decision-making. By synthesizing current trends and actionable recommendations, this document equips industry leaders with the intelligence needed to navigate a complex and rapidly changing landscape.
Revolutionary Technological and Therapeutic Advancements Reshaping How Dysmenorrhea Is Diagnosed, Managed, and Treated Across Medical and Wellness Sectors
Over the past two years, the dysmenorrhea treatment landscape has undergone a radical transformation driven by digital health innovations. Remote monitoring platforms now allow clinicians to track menstrual pain patterns in real time, while AI-powered mobile applications offer personalized pain management plans that adjust dosage recommendations based on symptom severity. Wearable sensors equipped with smart heat delivery systems have entered clinical trials, demonstrating potential to alleviate cramping without reliance on systemic analgesics. These technological breakthroughs are redefining standards of care, empowering patients with actionable insights and fostering data-driven treatment adaptation.
Concurrently, non-pharmacological therapies have gained unprecedented traction, spurred by mounting evidence and consumer preference for natural and minimally invasive interventions. Sophisticated heat therapy devices featuring targeted thermal mapping enable precise delivery of relief to the uterine region, complementing traditional approaches such as acupuncture therapy and structured exercise regimens. Meanwhile, the dietary supplements segment has diversified, with standardized herbal extracts, omega-3 fatty acids, and micronutrient formulations tailored to address inflammatory pathways implicated in menstrual pain. As a result, interdisciplinary protocols that integrate medical and wellness modalities have become more prevalent across clinics and specialty centers.
Moreover, strategic collaborations between pharmaceutical companies and digital therapeutics startups are reshaping clinical pathways, as regulators work to establish frameworks for the evaluation and reimbursement of software-based interventions. By leveraging real-world evidence and patient-reported outcomes, these partnerships aim to validate novel treatment combinations that blend hormonal contraceptives and smart devices, ultimately enhancing adherence and reducing adverse effects. This convergence of technology, personalized medicine, and integrated care models marks a pivotal shift, setting the stage for a more patient-centric approach to dysmenorrhea management.
Assessing the Ripple Effects of 2025 US Tariff Policies on Supply Chains, Pricing Dynamics, and Access to Dysmenorrhea Treatments Across Domestic Markets
In early 2025, new United States tariffs targeting imported pharmaceutical active ingredients and medical device components introduced a layer of complexity to the dysmenorrhea treatment supply chain. Aimed at bolstering domestic production and addressing trade imbalances, these measures have led to adjustments in procurement strategies among manufacturers and distributors. The levies apply to both raw materials essential for non-pharmacological device manufacturing and key intermediates used in the synthesis of hormonal contraceptives and nonsteroidal anti-inflammatory drugs.
The imposition of these tariffs has precipitated cascading effects on production costs and pricing dynamics. Manufacturers sourcing herbal extracts, omega-3 oils, and specialized polymers for heat therapy devices have faced margin compression, prompting cost pass-through considerations that risk impacting patient affordability. Simultaneously, generic drug producers reliant on established offshore API suppliers are exploring alternative arrangements to mitigate supply disruptions. This has accelerated partnerships with domestic chemical firms, spurred investment in in-house formulation capabilities, and fostered greater emphasis on supply chain resilience.
Consequently, industry stakeholders are recalibrating their operational models by pursuing vertical integration and diversifying supplier portfolios. Some technology-driven entrants have reshaped design specifications to minimize dependencies on tariff-sensitive components, while leading pharmaceutical companies are engaging in policy dialogues to advocate for exemption channels or streamlined import pathways for critical ingredients. In this environment, agility in procurement and proactive risk management are emerging as essential competencies, ensuring that patients retain uninterrupted access to a comprehensive range of dysmenorrhea treatments.
Unveiling Critical Patient and Product Segmentation Dimensions That Drive Market Dynamics and Guide Tailored Strategies for Dysmenorrhea Interventions
A nuanced understanding of treatment type segmentation is imperative, given the broad spectrum of approaches available for dysmenorrhea management. The market encompasses both non-pharmacological modalities and traditional pharmacological therapies. Within the former category, acupuncture therapy and exercise therapy have seen heightened clinical acceptance, while heat therapy devices leverage advanced materials science to deliver targeted relief. Dietary supplements also form a critical subcategory, with herbal extracts standardized for potency, omega-3 supplements researched for anti-inflammatory benefits, and vitamin supplements tailored to address micronutrient deficiencies. Meanwhile, pharmacological interventions bifurcate into hormonal contraceptives and nonsteroidal anti-inflammatory drugs, each offering distinct risk-benefit profiles. These treatments are administered across injectable, oral, and topical routes, reflecting diverse patient preferences and clinical scenarios.
Further granularity arises from how treatments reach end users and the contexts in which they are applied. Distribution channels span hospital pharmacy networks, brick-and-mortar pharmacies, and online pharmacy models, each presenting unique regulatory, reimbursement, and logistical considerations. Treatment application enters the equation as well, with primary dysmenorrhea patients often seeking first-line relief under established guidelines, while secondary dysmenorrhea cases demand more targeted diagnostic workups and adjunct interventions. The end-user landscape includes traditional hospital settings and specialized clinics, but also extends to home care environments where wearables and self-administered therapies gain traction. Specialty centers-whether focused on gynecological care or integrated fitness and wellness services-now serve as pivotal hubs for adopting comprehensive treatment regimens and driving patient engagement.
This comprehensive research report categorizes the Dysmenorrhea Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Drug Class
- Route Of Administration
- Distribution Channel
- Application
- End User
Decoding Regional Market Landscapes: Distinct Demands, Healthcare Infrastructure, and Growth Opportunities Across Americas, EMEA, and Asia-Pacific
In the Americas, the dysmenorrhea market is characterized by robust infrastructure for both pharmacological and complementary therapies, supported by comprehensive insurance frameworks and progressive telehealth adoption. Patients in the United States and Canada increasingly leverage digital health platforms to access remote consultations and prescription services, while the prevalence of home-care management solutions has risen in response to demand for convenience and privacy. Conversely, regulatory diversity across Europe, the Middle East, and Africa complicates market entry, as varying reimbursement policies and standards for clinical evidence influence adoption rates. Lessons learned in Western Europe are now informing initiatives in the Middle East, where government-led health programs are expanding access to both traditional NSAIDs and innovative non-pharmacological modalities.
Asia-Pacific, however, presents a blended landscape of high-growth emerging economies and mature markets, each demonstrating distinct consumer behaviors. In Japan and Australia, advanced healthcare systems facilitate rapid integration of smart heat therapy and digital therapeutics into mainstream care, often supported by national health coverage. Meanwhile, countries across Southeast Asia and South Asia are witnessing increased interest in culturally rooted non-pharmacological approaches, such as acupuncture coupled with dietary supplement regimens based on herbal medicine traditions. Government-led public health campaigns are promoting menstrual health education, driving uptake of both over-the-counter NSAIDs and physician-prescribed hormonal contraceptives, while rising disposable incomes are enabling broader adoption of premium wellness solutions and specialized clinic services.
This comprehensive research report examines key regions that drive the evolution of the Dysmenorrhea Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Disruptors: Strategic Initiatives, Portfolio Diversification, and Competitive Positioning in Dysmenorrhea Treatment Arena
Leading pharmaceutical manufacturers have responded to evolving dysmenorrhea dynamics by expanding their product portfolios to include both legacy analgesics and novel hormonal contraceptive formulations designed for improved tolerability and adherence. Several global players have invested in targeted research collaborations to optimize dosing regimens and minimize side effects, while forging alliances with device innovators to bundle smart heat patches or wearable sensors with prescription therapies. Meanwhile, established medical device companies are capitalizing on advances in materials science to launch next-generation thermal delivery systems capable of maintaining consistent therapeutic temperatures and integrating wireless monitoring capabilities.
At the same time, an influx of digital health startups is disrupting traditional market boundaries, introducing AI-driven symptom trackers, virtual coaching programs, and remote monitoring tools that complement core treatment offerings. Nutraceutical companies specializing in dietary supplements have carved out niche positions by standardizing herbal extract formulations and conducting clinical validation studies to substantiate anti-inflammatory claims. Cross-sector collaborations are gaining traction; for instance, partnerships between e-pharmacies and fitness-centric specialty centers are creating bundled wellness packages that combine exercise therapy regimens with curated supplement subscriptions. Collectively, these strategic initiatives underscore a broader trend toward integrated, patient-centric solutions that blur the lines between pharmaceuticals, devices, and digital services.
This comprehensive research report delivers an in-depth overview of the principal market players in the Dysmenorrhea Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- AbbVie Inc.
- Astellas Pharma
- AstraZeneca
- Bayer AG
- Boehringer Ingelheim
- Cipla Inc.
- Dr. Reddy's Laboratories Ltd.
- GlaxoSmithKline plc (GSK)
- Haleon Plc
- Himalaya Wellness Co.
- Johnson & Johnson
- Lupin
- Novartis International AG
- Perrigo Company plc
- Pfizer Inc.
- Reckitt Benckiser Group PLC.
- Sanofi S.A.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
Actionable Insights and Strategic Pathways for Industry Leaders to Navigate Shifting Policies, Patient Needs, and Competitive Pressures in Dysmenorrhea
Industry leaders should prioritize the integration of digital therapeutics and remote monitoring into core treatment portfolios, thereby augmenting traditional pharmacological and non-pharmacological offerings with real-time data analytics and personalized care pathways. Building strategic partnerships with device manufacturers and software developers can accelerate time to market for integrated solutions, while diversifying supplier networks will mitigate the impact of tariff-related supply disruptions. Tailoring product formulations and delivery mechanisms to the distinct needs of primary versus secondary dysmenorrhea patients-and aligning route-of-administration options with patient lifestyles-will deepen engagement and foster adherence across demographic segments.
In parallel, organizations must strengthen clinical validation efforts by investing in robust evidence-generation studies that evaluate combination therapies and digital interventions within decentralized trial frameworks. Proactive engagement with policymakers and payers can facilitate favorable reimbursement conditions for innovative modalities, while targeted patient education campaigns will raise awareness of emerging treatment alternatives and improve self-management capabilities. Finally, adopting region-specific go-to-market strategies-attuned to local regulatory environments, cultural preferences, and channel infrastructure-will enable sustained growth in diverse markets, from mature Western economies to high-potential Asia-Pacific jurisdictions.
Rigorous and Transparent Research Methodology: Integrating Qualitative, Quantitative, and Stakeholder Perspectives to Ensure Comprehensive Market Intelligence
This research employed a rigorous and transparent methodology, beginning with an extensive review of peer-reviewed literature, clinical guidelines, and regulatory filings to establish a foundational understanding of dysmenorrhea treatment paradigms. Secondary data sources encompassed medical journals, patent databases, and industry white papers, which were systematically analyzed to identify prevailing trends and technological innovations. Complementing this, primary research entailed in-depth interviews with key stakeholders-including gynecologists, pain management specialists, device engineers, and patient advocacy representatives-to capture diverse perspectives on treatment efficacy, market access challenges, and emerging unmet needs.
Quantitative data were synthesized through cross-sectional surveys distributed to healthcare providers and consumers, yielding insights into prescribing behaviors, therapy adoption rates, and patient satisfaction metrics. Data triangulation techniques were applied to reconcile findings from multiple channels, ensuring consistency and reliability. The resulting intelligence underwent validation via expert panel reviews, wherein an advisory board of clinicians and industry veterans assessed methodological soundness and interpretive accuracy. This multi-layered approach underpins the comprehensive and actionable insights presented throughout this executive summary.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Dysmenorrhea Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Dysmenorrhea Treatment Market, by Treatment Type
- Dysmenorrhea Treatment Market, by Drug Class
- Dysmenorrhea Treatment Market, by Route Of Administration
- Dysmenorrhea Treatment Market, by Distribution Channel
- Dysmenorrhea Treatment Market, by Application
- Dysmenorrhea Treatment Market, by End User
- Dysmenorrhea Treatment Market, by Region
- Dysmenorrhea Treatment Market, by Group
- Dysmenorrhea Treatment Market, by Country
- United States Dysmenorrhea Treatment Market
- China Dysmenorrhea Treatment Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1590 ]
Synthesis of Key Findings and Strategic Implications for Decision-Makers Addressing Dysmenorrhea Challenges in a Rapidly Evolving Healthcare Environment
The landscape of dysmenorrhea treatment is undergoing a pivotal transformation, shaped by converging forces in technology, policy, and patient empowerment. From the advent of AI-driven symptom trackers and next-generation heat therapy devices to the recalibrated supply chain dynamics spurred by 2025 tariff measures, stakeholders must adapt to an increasingly integrated and patient-centric paradigm. By leveraging detailed segmentation insights and regional nuances, industry participants can tailor offerings that resonate with distinct patient cohorts, address unmet needs, and capitalize on evolving reimbursement frameworks.
Looking ahead, the synthesis of strategic partnerships, clinical validation, and robust patient engagement will be critical to sustaining innovation and driving market growth. Navigating this complexity demands agility, as well as a deep appreciation for the diverse pathways through which dysmenorrhea is experienced and managed. Ultimately, the insights and recommendations outlined in this executive summary equip decision-makers with the actionable intelligence required to chart a clear course. By embracing a holistic, data-driven approach, organizations can unlock new opportunities to enhance patient outcomes and reshape the future of menstrual health management.
Engage with Our Lead Associate Director, Ketan Rohom, to Secure Comprehensive Dysmenorrhea Market Insights and Drive Informed Strategic Decisions
For decision-makers seeking a deeper dive into the dynamics of the dysmenorrhea market, we invite you to engage directly with Ketan Rohom, Associate Director of Sales & Marketing. With extensive experience in guiding healthcare organizations through complex market landscapes, Ketan can provide tailored guidance on leveraging the full breadth of insights contained within the complete report. Whether you require custom segmentation analysis, detailed competitive benchmarking, or hands-on strategic advisory, he stands ready to facilitate access to the proprietary research and expert counsel that will inform your next steps.
Secure your copy of the comprehensive dysmenorrhea market research report today to gain exclusive access to in-depth intelligence on emerging treatment innovations, policy and tariff implications, regional growth levers, and actionable recommendations. By partnering with our lead associate director, you will equip your organization with the clarity and foresight needed to optimize product strategies, navigate regulatory shifts, and deliver differentiated value to patients. Contact Ketan directly to explore flexible licensing options and ensure your team is positioned at the forefront of this rapidly evolving healthcare segment.

- How big is the Dysmenorrhea Treatment Market?
- What is the Dysmenorrhea Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




