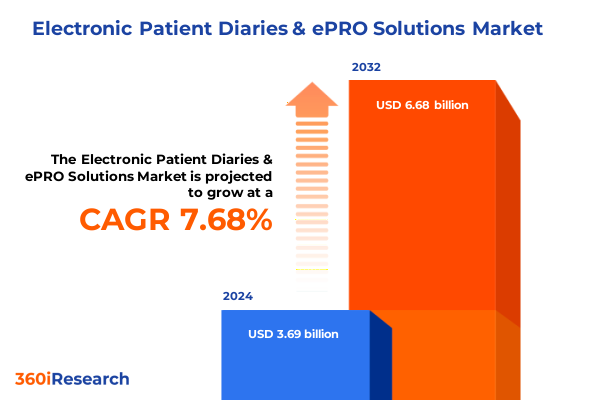
The Electronic Patient Diaries & ePRO Solutions Market size was estimated at USD 3.95 billion in 2025 and expected to reach USD 4.23 billion in 2026, at a CAGR of 7.79% to reach USD 6.68 billion by 2032.
How digital patient diaries are revolutionizing clinical trials by enhancing data accuracy, patient engagement, and operational efficiency
Clinical development programs worldwide are experiencing a profound transformation as patient-centered data collection moves from paper-based questionnaires and manual diaries to fully digitized platforms. Digital patient diaries empower sponsors to capture real-time, high-fidelity patient-reported outcomes directly from individuals, significantly reducing recall bias and enhancing the quality of longitudinal data. With growing pressure to accelerate trial timelines, contain costs, and comply with increasingly stringent regulatory requirements, electronic diaries and ePRO systems have emerged as critical enablers of more efficient, patient-centric studies.
Advancements in mobile technology, remote connectivity, and user-centric design have catalyzed the rapid uptake of digital diary solutions. Patients can now report symptoms, adverse events, and quality-of-life measures through intuitive applications on smartphones and web portals. Meanwhile, trial sites and sponsors benefit from automated data validation, secure cloud storage, and near–real-time dashboards that streamline monitoring and decision making. This shift toward digital-first patient engagement fundamentally alters the interplay between stakeholders, enhancing transparency, improving adherence, and supporting decentralized trial models.
This executive summary presents a structured analysis of the forces reshaping the ePRO landscape, from macroeconomic influences and regulatory shifts to segmentation-driven insights and regional dynamics. It highlights the cumulative impact of United States tariffs enacted in 2025, details critical best practices distilled from key industry participants, and provides a clear path forward through strategic recommendations. By the end of this overview, decision makers will possess a concise yet comprehensive understanding of how to harness electronic patient diaries to deliver more meaningful, cost-effective clinical outcomes.
Exploring pivotal industry shifts such as artificial intelligence, remote monitoring, and interoperability reshaping electronic patient diaries and ePRO systems
The electronic patient diary and ePRO sector has undergone seismic shifts as new technologies converge with evolving stakeholder expectations. Artificial intelligence and machine learning algorithms now augment data quality checks, identifying anomalies and compliance risks in near real time. These intelligent tools not only reduce manual query resolution but also drive deeper insights by correlating patient-reported data with sensor-based biometric measurements. As a result, sponsors can detect safety signals and efficacy trends earlier in the study lifecycle, enabling proactive risk mitigation.
Concurrently, the integration of remote monitoring solutions has expanded the traditional boundaries of clinical research. Wearable devices and mobile health sensors seamlessly feed physiological data-such as heart rate variability and activity levels-into ePRO platforms. This convergence fosters a holistic view of patient health, wherein subjective self-reports are contextualized by objective metrics. The synergy between passive monitoring and active diary entries generates richer datasets, supporting more nuanced endpoint analyses and adaptive trial designs.
Interoperability has also emerged as a transformative force, driven by the need for seamless data exchange across electronic health records, clinical trial management systems, and patient engagement portals. Standardized data models and application programming interfaces facilitate end-to-end workflows, from patient enrollment through database lock. As connectivity improves, sponsors can orchestrate multi-vendor ecosystems while preserving data integrity and regulatory compliance, thereby unlocking new opportunities for decentralized and hybrid trial architectures.
Assessing the multifaceted effects of United States 2025 tariff implementations on supply chains, vendor pricing, and adoption timelines in digital health solutions
In 2025, the United States implemented a series of tariff measures targeting hardware imports and digital goods critical to decentralized clinical trials. Although the overarching goal was to bolster domestic manufacturing, these levies have exerted multifaceted pressures on the electronic patient diary market. Manufacturers of desktop terminals and wearable devices have experienced increased component costs, prompting a realignment of supply chains toward North American and nearshore vendors. While this shift supports local economies, trial sponsors must now navigate extended lead times and higher per-unit expenses when provisioning hardware for global studies.
Software and service providers have also felt the ripple effects of U.S. tariff policies. Implementation services, including integration and training and support, often rely on skilled technical professionals whose labor rates may rise in response to increased operational costs. Meanwhile, platform and software solution vendors have adjusted pricing structures to offset hardware-associated revenue impacts. These adjustments have led to tighter budgetary constraints for study start-up and site activation, particularly for mid-sized biotechs and contract research organizations with limited financial flexibility.
To manage these challenges, industry participants are exploring strategic partnerships and bulk procurement agreements to stabilize pricing. Some sponsors are pivoting toward cloud-based hardware-as-a-service models that shift capital expenditures into operational budgets. Others are renegotiating service-level agreements to include tariff pass-through clauses and flexible delivery schedules. By taking a proactive stance on supply chain diversification and contractual agility, clinical trial stakeholders can mitigate the adverse effects of tariff-related cost inflation.
Unveiling critical insights from market segmentation across product types, components, deployment modes, end users, and therapeutic areas within ePRO ecosystems
The product landscape for electronic patient diaries and ePRO systems is defined by a dual focus on service delivery and software platforms. Within the service domain, consulting engagements guide organizations through solution selection, regulatory navigation, and change management, while training and support offerings ensure end users can adopt digital diaries effectively. On the software side, mobile-based applications cater to smartphone and tablet users, delivering push notifications and offline data capture capabilities, whereas web-based portals provide browser-agnostic access for patients who prefer desktop interactions or study sites that centralize data review workflows.
Component-level analysis underscores the role of desktop terminals and wearable devices in capturing objective health metrics, balanced by a robust infrastructure of implementation services that spans systems integration and ongoing training. Platform solutions consolidate data ingestion, storage, and analytics within unified environments, while specialized software solutions focus on customizable survey modules and advanced reporting functions. This layered approach allows stakeholders to tailor their technology stacks, selecting hardware elements that enhance patient engagement and back-end services that reinforce data integrity and compliance standards.
Deployment considerations further distinguish market offerings between cloud and on-premises models. Cloud environments deliver scalability and rapid provisioning, supporting remote and decentralized trial designs, while on-premises installations appeal to organizations with strict data residency or security mandates. End users-including contract research organizations, healthcare providers, and pharmaceutical and biotechnology firms-leverage these deployment options according to their operational footprints, regulatory obligations, and internal IT capabilities.
Therapeutic area specialization remains a critical axis of differentiation. Cardiovascular studies often demand high-frequency symptom diaries and integration with wearable devices for heart monitoring. Neurology trials benefit from cognitive assessment modules that capture patient perceptions of functional status, while oncology protocols require nuanced quality-of-life measurements to track treatment tolerability. By aligning segmentation insights with therapeutic priorities, sponsors can optimize solution selection and enhance endpoint relevance across diverse clinical programs.
This comprehensive research report categorizes the Electronic Patient Diaries & ePRO Solutions market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Component
- Therapeutic Area
- Deployment Mode
- End User
Analyzing regional dynamics across Americas, EMEA, and Asia-Pacific revealing unique adoption patterns and strategic opportunities for electronic patient diary solutions
The Americas region continues to lead in the adoption of electronic patient diaries and ePRO solutions, driven by robust investment in clinical research infrastructure and a regulatory environment that encourages digital innovation. North American sponsors capitalize on established telehealth networks and widespread smartphone penetration to enroll diverse patient cohorts remotely. Latin American countries are rapidly augmenting their research capabilities, supported by strategic partnerships between multinational sponsors and regional contract research organizations that facilitate technology transfer and localized training initiatives.
In Europe, Middle East, and Africa, varying degrees of regulatory harmonization and digital health maturity shape regional dynamics. Western European markets boast comprehensive eHealth frameworks and standardized patient-reported outcome guidelines, enabling seamless integration of digital diaries. Meanwhile, emerging markets in Eastern Europe and select Gulf Cooperation Council nations are scaling pilot programs, emphasizing interoperability and local-language support. Africa presents both challenges and opportunities, with infrastructure gaps offset by high levels of mobile connectivity that underpin innovative decentralized trial models.
Asia-Pacific displays a heterogeneous landscape where advanced economies like Japan, South Korea, and Australia lead in clinical digitization, leveraging national health data exchanges and regulatory incentives to drive ePRO initiatives. Southeast Asian nations are investing in public–private collaborations to modernize trial processes, while China’s evolving regulatory pathways and growing domestic clinical pipeline signal increasing demand for sophisticated patient-reported outcome tools. Across the region, cultural nuances and language diversity necessitate adaptable user interfaces and tailored engagement strategies to ensure broad patient participation.
This comprehensive research report examines key regions that drive the evolution of the Electronic Patient Diaries & ePRO Solutions market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting leading industry players, their innovative offerings, strategic partnerships, and competitive differentiators within the electronic patient diary and ePRO market
Leading providers in the electronic patient diary and ePRO space are distinguished by their integrated technology stacks, global service networks, and strategic partnerships. Established clinical technology vendors have expanded their offerings to include AI-driven analytics modules and interoperability suites that connect seamlessly with electronic health records and wearable ecosystems. These organizations often differentiate themselves through extensive regulatory expertise and end-to-end implementation services that address the complexities of multinational trials.
Innovative pure-play ePRO specialists emphasize agility and user experience, deploying rapid configuration engines and modular architecture that enable sponsors to customize diary schedules, notification schemes, and reporting dashboards with minimal development overhead. Strategic alliances with device manufacturers and telemedicine platforms enhance the value proposition by delivering holistic patient monitoring solutions. Additionally, several contract research organizations have internalized ePRO capabilities, bundling diary management, site training, and patient support as part of their full-service clinical trial portfolios.
Competitive pressures have also spurred consolidation and cross-sector collaboration. Partnerships between software vendors and data analytics firms are creating more robust end-to-end offerings, blending advanced patient engagement features with predictive modeling and real-world evidence generation. As market dynamics mature, vendors are investing in interoperability certifications and global data privacy compliance to reinforce trust and simplify technology adoption for multinational sponsors.
This comprehensive research report delivers an in-depth overview of the principal market players in the Electronic Patient Diaries & ePRO Solutions market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Anju Software, Inc.
- ArisGlobal LLC
- Castor EDC
- Clario
- Climedo Health GmbH
- ClinCapture, Inc.
- Dassault Systèmes SE
- eClinical Solutions, LLC
- Health Diary, Inc.
- ICON plc
- Illingworth Research Group, Ltd.
- IQVIA Holdings, Inc.
- Kayentis SAS
- MedNet Solutions, Inc.
- OmniComm Systems, Inc.
- Oracle Corporation
- Parexel International Corporation
- SAS Institute Inc.
- SciBase AB
- Science 37, Inc.
- Signant Health, Inc.
- The Diary Pte Ltd
- TransPerfect Translations International, Inc.
- Y-Prime, LLC
Strategic recommendations for industry leaders to harness digital diary technologies, optimize patient engagement, and achieve sustainable competitive advantage
Industry leaders should prioritize interoperability and data integration as foundational elements of their digital patient diary strategies, ensuring seamless connectivity between ePRO platforms, electronic health records, and external biometric devices. By adopting open application programming interfaces and standardized data schemas, organizations can reduce vendor lock-in and accelerate study start-up by streamlining data transactions across disparate systems. Such integration efforts not only improve operational efficiency but also enhance the granularity of insights derived from patient-reported outcomes.
Investing in patient-centric design is equally essential; intuitive user interfaces and adaptive questionnaire algorithms can significantly boost compliance rates and data completeness. Incorporating real-time feedback mechanisms and personalized reminders helps maintain engagement, while multilingual support and accessibility features broaden demographic reach. Embedding educational resources within digital diaries empowers participants to understand trial objectives, fostering trust and reducing dropout rates.
Stakeholders must also build robust training and change management frameworks, enabling site staff and patients to navigate new technologies confidently. Collaborative workshops and digital onboarding modules can address common adoption barriers, while clear governance structures ensure accountability and rapid escalation of technical issues. Finally, establishing cross-functional innovation councils that include clinical operations, IT, and patient engagement teams will facilitate continuous refinement, allowing organizations to stay ahead of emerging trends and regulatory developments in the evolving ePRO landscape.
Comprehensive overview of the research design, data sources, validation processes, and analytical frameworks underpinning the electronic patient diary market study
The research methodology underpinning this study combines rigorous primary and secondary approaches to deliver an unbiased, comprehensive market assessment. Secondary research sources include peer-reviewed journals, regulatory agency guidelines, and technology white papers, ensuring a solid theoretical foundation and alignment with current best practices. Primary insights were collected through in-depth interviews with clinical operations executives, technology providers, and patient advocacy group representatives, offering firsthand perspectives on solution implementation challenges and success factors.
Quantitative data analyses employed vendor profiling and technology landscape mapping, categorizing offerings across product types, components, deployment modes, end users, and therapeutic areas. Each vendor’s capabilities were evaluated against standardized criteria for functionality, scalability, and regulatory compliance. Qualitative assessments complemented these findings by exploring user experience, integration complexity, and support frameworks, providing a holistic view of the competitive environment.
To ensure validity and reliability, the study utilized data triangulation techniques, cross-verifying information from interviews, published reports, and real-world case studies. Rigorous peer review processes and expert panel consultations validated key assumptions and analytical models. Ethical considerations and data privacy protocols were strictly adhered to, particularly when handling patient-derived information and proprietary vendor data, ensuring the integrity of all research outputs.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Electronic Patient Diaries & ePRO Solutions market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Electronic Patient Diaries & ePRO Solutions Market, by Product Type
- Electronic Patient Diaries & ePRO Solutions Market, by Component
- Electronic Patient Diaries & ePRO Solutions Market, by Therapeutic Area
- Electronic Patient Diaries & ePRO Solutions Market, by Deployment Mode
- Electronic Patient Diaries & ePRO Solutions Market, by End User
- Electronic Patient Diaries & ePRO Solutions Market, by Region
- Electronic Patient Diaries & ePRO Solutions Market, by Group
- Electronic Patient Diaries & ePRO Solutions Market, by Country
- United States Electronic Patient Diaries & ePRO Solutions Market
- China Electronic Patient Diaries & ePRO Solutions Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1749 ]
Synthesis of key findings emphasizing market maturity, emerging trends, and critical considerations for stakeholders in the electronic patient diary and ePRO domain
The analysis of electronic patient diaries and ePRO solutions reveals a mature yet rapidly evolving landscape shaped by technological innovation, regulatory pressure, and heightened patient expectations. The convergence of artificial intelligence, remote monitoring, and interoperability frameworks has enabled stakeholders to collect richer, more accurate patient-reported data while optimizing trial efficiency. At the same time, regional and tariff-related considerations underscore the importance of adaptive sourcing strategies and flexible deployment models.
Segmentation insights demonstrate that sponsors must tailor their approaches based on service and software preferences, component priorities, deployment requirements, and therapeutic area nuances. Regional dynamics further highlight the need for localized engagement tactics and compliance strategies, while key market participants showcase the competitive advantages of integrated ecosystems and user-focused design. By synthesizing these findings, industry leaders can navigate complexity with clarity, driving more effective clinical outcomes and patient experiences.
Ultimately, the strategic recommendations offered herein provide a roadmap for organizations to elevate their digital patient diary initiatives, from establishing interoperability foundations to cultivating patient-centric interfaces and reinforcing governance structures. As the ePRO domain continues to advance, stakeholders who embrace these insights will be best positioned to lead the next wave of innovation in clinical research.
Secure personalized guidance from Associate Director Ketan Rohom for actionable insights into electronic patient diaries and ePRO solutions
To gain an unparalleled understanding of electronic patient diaries and ePRO solutions and leverage them to optimize clinical trial execution, connect directly with Associate Director Ketan Rohom, who can provide a bespoke overview of study design implications, technology selection, and integration pathways. Engaging with an expert at this level ensures that your organization can harness the full spectrum of insights contained within the comprehensive market research report. Reach out today to secure tailored support and actionable guidance that aligns your strategic objectives with the latest innovations and best practices in patient-reported outcome management.

- How big is the Electronic Patient Diaries & ePRO Solutions Market?
- What is the Electronic Patient Diaries & ePRO Solutions Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




