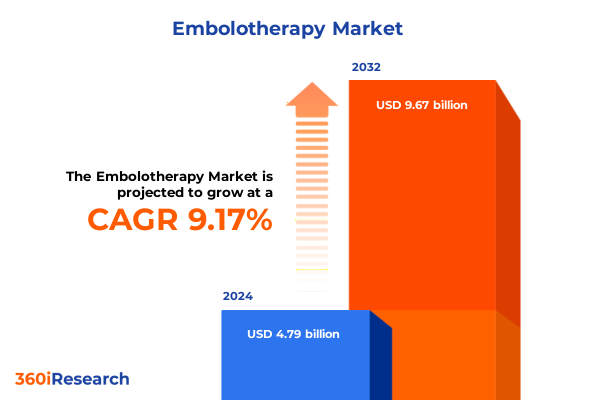
The Embolotherapy Market size was estimated at USD 5.22 billion in 2025 and expected to reach USD 5.68 billion in 2026, at a CAGR of 9.19% to reach USD 9.67 billion by 2032.
Unveiling the Evolution and Significance of Embolotherapy as a Revolutionary Cornerstone of Minimally Invasive Vascular Interventions
Embolotherapy has emerged as one of the most transformative minimally invasive interventions in modern medicine, offering clinicians the ability to precisely occlude pathological blood vessels to treat a variety of conditions with reduced patient morbidity. Leveraging advanced imaging modalities and catheter-based delivery systems, this therapeutic approach deploys embolic agents directly to target sites, thereby preserving surrounding healthy tissue and enhancing procedural safety. Recent advancements in high-definition fluoroscopy, MRI guidance, and three-dimensional angiography have markedly improved the visualization of vascular architecture, enabling more accurate navigation of microcatheters and proportional deployment of agents such as microspheres, microspheres loaded with chemotherapeutics, or liquid embolics. These innovations not only elevate the technical precision of embolization procedures but also broaden the scope of clinical applications, reinforcing embolotherapy’s role as a cornerstone in interventional radiology.
Moreover, the evolution of embolic materials-from conventional polyvinyl alcohol particles to drug-eluting beads and biodegradable microspheres-has introduced dual-function capabilities that combine mechanical occlusion with localized pharmacotherapy. Such advancements allow for sustained drug release at the occlusion site, fostering improved antitumor efficacy and reduced systemic toxicity. As catheter materials and microcatheter designs have evolved to become more flexible and steerable, interventionalists can now traverse complex vascular networks in regions like the neurovasculature and peripheral limb arteries, further diversifying embolotherapy’s clinical impact. In light of these developments, embolotherapy continues to attract significant attention for its minimally invasive profile, clinical versatility, and capacity to address conditions ranging from arteriovenous malformations to trauma-induced hemorrhage.
Exploring the Pivotal Technological and Clinical Shifts Reshaping the Embolotherapy Landscape Across Diverse Therapeutic Domains
The embolotherapy landscape has witnessed a series of pivotal technological and clinical shifts that are redefining treatment paradigms across multiple therapeutic domains. Innovations in catheter design, such as steerable microcatheters, have significantly improved access to tortuous vessels, enabling safer and more effective delivery of embolic materials. Concurrently, the integration of drug-eluting beads and biodegradable microspheres has transformed traditional embolic occlusion by incorporating localized chemotherapeutic or antiangiogenic therapy, thus enhancing therapeutic outcomes for oncology patients. These material-based advancements, paired with real-time imaging enhancements like high-resolution fluoroscopy and MRI-guided overlays, are accelerating procedural precision and minimizing non-target embolization.
Additionally, the emergence of robotic-assisted navigation in embolotherapy procedures is beginning to streamline complex interventions by reducing operator fatigue and radiation exposure. Meanwhile, artificial intelligence and machine learning algorithms are being developed to predict procedural outcomes and optimize agent delivery strategies, offering personalized treatment planning capabilities that were previously unattainable. On the clinical front, expanded indications such as uterine fibroid embolization, transarterial radioembolization for hepatic malignancies, and refractory gastrointestinal bleeding underscore the modality’s versatility. At the same time, refined embolotherapy protocols in neurovascular applications-targeting conditions like arteriovenous malformations, cerebral aneurysms, and intracranial tumors-are benefiting from more precise agent formulations and deployment techniques.
Analyzing the Far-reaching Cumulative Impact of United States 2025 Tariff Policies on the Embolotherapy Supply Chain and Clinical Practice
United States tariff policies implemented in 2025 under the Section 301 framework have introduced substantial duties on a wide range of imported medical products, directly affecting the embolotherapy supply chain. For instance, tariffs on surgical and non-surgical respirators and facemasks increased to 25 percent in late 2024, while syringes and needles, crucial for catheter priming and agent injection, face duties as high as 100 percent for certain subcategories currently excluded, with additional increases scheduled by 2026. These measures, coupled with earlier impositions of 50 percent duties on syringes and 25 percent on medical gloves and critical minerals, have cumulatively elevated import costs for devices and components integral to embolotherapy procedures.
In parallel, GlobalData has highlighted that proposed expansions of Section 301 tariffs on Class I and II medical devices could further destabilize global supply chains, particularly for minimally invasive instruments used in neurovascular and peripheral embolization, by amplifying procurement costs and elongating lead times. Companies heavily reliant on Chinese manufacturing or raw material sourcing must now evaluate alternative production locations or dual-source arrangements to mitigate these emerging trade risks. Consequently, healthcare providers are likely to encounter higher expenditure on consumables such as microcatheters, embolic agents, and accessory devices, placing pressure on budget-constrained hospitals and specialty centers to offset the increased expense while maintaining procedural accessibility and patient safety.
Uncovering Critical Segmentation Insights Reflecting Product Type Application and End User Dynamics in the Embolotherapy Market
A comprehensive understanding of product type segmentation reveals that each category of embolic material brings distinct clinical and operational dynamics. Balloons facilitate temporary vessel occlusion with high precision, ideal for proximal vessel control, whereas detachable and pushable coils provide durable mechanical occlusion for aneurysms and arteriovenous malformations, benefiting from newer hydrogel formulations that promote vessel wall apposition. Liquid embolics such as ethylene vinyl alcohol copolymer systems offer superior penetration into complex vascular beds, proving indispensable in neurovascular and peripheral hemorrhage settings. Particle embolics, including calibrated microspheres, deliver embolization with predictable distal distribution and are commonly employed in transarterial chemoembolization and radioembolization treatments. Plugs serve as robust occlusive devices in large vessel applications, complementing coil systems and expanding therapeutic options across the embolotherapy spectrum.
By application, the neurovascular embolization segment-encompassing arteriovenous malformation management, cerebral aneurysm coiling, and tumor embolization-continues to drive innovation through refined agent formulations and imaging enhancements. Simultaneously, peripheral arterial and venous embolization techniques have diversified trauma and gastrointestinal bleeding management, while prostate artery embolization emerges as an effective alternative for benign prostatic hyperplasia. Transarterial chemoembolization and radioembolization maintain central roles in hepatic oncology, and uterine fibroid embolization offers a non-surgical solution for symptomatic fibroids, reflecting embolotherapy’s broad therapeutic reach.
Examining end user segmentation underscores that ambulatory surgical centers are increasingly adopting embolization procedures due to their minimally invasive profile and cost efficiencies, while hospitals remain the primary providers for complex interventions requiring multidisciplinary support. Specialty clinics, particularly oncology centers and dedicated vascular centers, are harnessing targeted embolotherapy portfolios to enhance patient throughput and clinical outcomes, highlighting the modality’s adaptability across diverse care settings.
This comprehensive research report categorizes the Embolotherapy market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- End User
Delving into Key Regional Dynamics to Illuminate the Americas Europe Middle East Africa and Asia Pacific Embolotherapy Landscape
In the Americas, especially within the United States and Canada, advanced reimbursement frameworks and high per capita healthcare expenditure have fostered rapid adoption of novel embolization technologies. Leading centers of interventional radiology are at the forefront of integrating robotic navigation and AI-driven planning tools, while collaborative research networks between academic institutions and industry partners accelerate the translation of next-generation embolic agents into clinical practice. Latin American markets, although constrained by budgetary limitations, are demonstrating gradual uptake of cost-effective coil and particle systems, supported by targeted training initiatives and public–private partnerships.
Europe, the Middle East, and Africa present a heterogeneous landscape. Western Europe benefits from well-established regulatory pathways and centralized procurement models, enabling efficient introduction of advanced embolic platforms. Meanwhile, in regions such as the Gulf Cooperation Council and South Africa, strategic investments in healthcare infrastructure and bespoke reimbursement schemes are boosting the availability of neurovascular and peripheral embolization services. Conversely, some markets in Eastern Europe and sub-Saharan Africa face challenges related to supply chain logistics and limited specialist training, which temper the expansion of embolotherapy offerings.
Asia-Pacific exhibits one of the highest growth trajectories, driven by increasing healthcare budgets, rising prevalence of vascular and oncological conditions, and government-led initiatives to modernize medical infrastructure. Japan’s aging population continues to rely on embolization for hepatic and uterine indications, while China’s extensive hospital networks are scaling TACE and TARE programs. Emerging economies such as India and Southeast Asian nations are also embracing low-cost coil systems and liquid embolics, facilitated by domestic manufacturing partnerships and capacity-building endeavors, ultimately broadening patient access to minimally invasive vascular interventions.
This comprehensive research report examines key regions that drive the evolution of the Embolotherapy market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Leading Industry Players and Their Strategic Innovations Driving Growth and Competition in the Global Embolotherapy Sector
Market-leading companies are spearheading innovation across the embolotherapy domain through clinical trials, product enhancements, and strategic collaborations. Medtronic’s Onyx™ liquid embolic system is currently under evaluation in a multi-center U.S. pivotal IDE study for peripheral arterial hemorrhage, marking a significant expansion of its neurovascular portfolio into peripheral applications. Johnson & Johnson’s TRUFILL n-BCA continues to serve as a benchmark in liquid embolic therapy for cerebral arteriovenous malformations, while Terumo’s MicroVention subsidiary maintains momentum with its PHIL™ embolic system, which benefits from a Humanitarian Use Device designation for neurovascular interventions.
In the coil segment, Terumo Medical Corporation’s AZUR HydroPack™ peripheral coil system, with its hydrogel-infused core, offers extended occlusion durability and simplified inventory management, positioning it as a differentiated solution in endovascular embolization. Other major players such as Cook Medical, Boston Scientific, and Balt Group continue to reinforce their coil and catheter portfolios through incremental product updates and distributor partnerships. Additionally, companies like Penumbra and Stryker are broadening their embolic agent and microcatheter capabilities, underscoring an intensifying competitive landscape where strategic R&D investments and regulatory approvals are critical to maintaining market leadership.
This comprehensive research report delivers an in-depth overview of the principal market players in the Embolotherapy market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Artio Medical Inc.
- B. Braun Melsungen AG
- Balt Extrusion SA
- Boston Scientific Corporation
- Cardinal Health Inc.
- Cook Medical LLC
- Embolx Inc.
- EndoShape Inc.
- Guerbet SA
- Instylla Inc.
- Johnson & Johnson
- Kaneka Corporation
- Medtronic plc
- Medtronic plc
- Merit Medical Systems Inc.
- Penumbra Inc.
- QT Vascular Ltd.
- Shape Memory Medical Inc.
- Sirtex Medical Limited
- Stryker Corporation
- Surefire Medical Inc.
- Terumo Corporation
- TriSalus Life Sciences Inc.
- Vascular Solutions Inc.
Strategic Actionable Recommendations to Empower Industry Leaders in Navigating Emerging Trends and Market Disruptions within Embolotherapy
Industry leaders should consider diversifying their supply chains to mitigate the impact of escalating tariffs and geopolitical tensions. By establishing alternative manufacturing bases or engaging in dual-sourcing strategies, companies can reduce dependency on single-country suppliers and maintain uninterrupted access to critical materials. Additionally, investing in domestic production capabilities for key consumables-such as catheter components and embolic agent precursors-can provide long-term cost stability and resilience against future trade policy shifts.
Moreover, prioritizing the integration of advanced imaging and AI-driven procedural planning tools will differentiate product portfolios and optimize clinical workflows. Collaborative ventures with software developers and academic research centers can accelerate the development of predictive outcome models, elevating patient safety and enhancing treatment efficacy. In parallel, expanding indication portfolios through targeted IDE studies and regulatory submissions-particularly in peripheral applications and emerging oncological targets-will unlock new revenue streams and broaden market penetration. Finally, fostering value-based care models by demonstrating health-economic benefits, such as reduced hospital stays and lower procedural complications, can strengthen payer partnerships and facilitate favorable reimbursement policies.
Outlining a Rigorous Research Methodology Incorporating Primary Insights Secondary Data and Triangulation Techniques for Comprehensive Embolotherapy Analysis
This research employed a rigorous multi-phase methodology to ensure the accuracy and comprehensiveness of insights. The process began with an exhaustive secondary research phase, leveraging peer-reviewed journal articles, clinical trial registries, and authoritative trade publications to map technological advancements and procedural trends. Simultaneously, regulatory filings and policy documents-such as U.S. Trade Representative Section 301 announcements-were analyzed to quantify tariff impacts on medical devices and consumables.
In the primary research phase, structured interviews were conducted with interventional radiologists, vascular surgeons, and industry executives to validate key findings and capture firsthand perspectives on market dynamics. Triangulation of data from secondary sources, expert opinions, and publicly available financial disclosures enabled robust cross-verification of thematic insights. Advanced analytical techniques, including segmentation modeling across product types, clinical applications, and end users, were applied to delineate market heterogeneity. Finally, regional and company-level analyses synthesized supply chain factors, competitive strategies, and regulatory landscapes to support actionable recommendations, ensuring the research’s relevance for decision-makers across the embolotherapy ecosystem.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Embolotherapy market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Embolotherapy Market, by Product Type
- Embolotherapy Market, by Application
- Embolotherapy Market, by End User
- Embolotherapy Market, by Region
- Embolotherapy Market, by Group
- Embolotherapy Market, by Country
- United States Embolotherapy Market
- China Embolotherapy Market
- Competitive Landscape
- List of Figures [Total: 15]
- List of Tables [Total: 1113 ]
Synthesizing Core Findings to Provide a Concise Strategic Outlook on Opportunities Challenges and Future Directions for Embolotherapy
The synthesis of clinical innovations, policy developments, and competitive strategies underscores embolotherapy’s dynamic evolution and its growing significance in minimally invasive vascular care. Technological breakthroughs in catheter navigation, imaging modalities, and embolic agent formulations are enabling more precise and effective treatments, while the expansion into new therapeutic areas-from hepatic oncology to peripheral hemorrhage-continues to broaden the modality’s scope. Policy shifts, notably the 2025 tariff increases, present both challenges and strategic imperatives, compelling stakeholders to fortify supply chains and diversify sourcing arrangements.
Segmentation analysis reveals distinct drivers and opportunities across product types, clinical applications, and care settings, highlighting areas where targeted investments can yield the greatest clinical and commercial returns. Regional insights further illustrate the importance of tailoring market strategies to local reimbursement frameworks, infrastructure capabilities, and regulatory pathways. Concurrently, leading companies are differentiating through clinical trials, novel material technologies, and strategic partnerships, setting new benchmarks for innovation and market performance.
Looking ahead, the convergence of AI-driven procedural planning, value-based contracting, and resilient supply chain models will define the next wave of growth in embolotherapy. By aligning R&D priorities with real-world clinical needs and regulatory landscapes, stakeholders can optimize patient outcomes, drive adoption, and sustain competitive advantage in this rapidly evolving field.
Engage with Ketan Rohom to Access Exclusive Embolotherapy Market Research Insights and Drive Strategic Decisions with Tailored Expertise
To obtain unparalleled insights into the global embolotherapy market and empower your strategic decisions with data-driven precision, reach out to Ketan Rohom, Associate Director of Sales & Marketing at 360iResearch. With his comprehensive understanding of procedural trends and regulatory influences, you can secure access to the full market research report that delves deeper into the nuanced segmentation, regional dynamics, and competitive landscape. Engaging directly with Ketan ensures you receive tailored guidance on leveraging emerging opportunities, mitigating supply chain challenges, and optimizing product portfolios for maximum clinical impact and commercial success. Partner with Ketan to explore customizable data packages, expert briefings, and interactive dashboards designed to integrate seamlessly with your planning processes. Elevate your market intelligence today and stay ahead of industry shifts by connecting with a dedicated specialist who can translate complex analyses into actionable strategies.

- How big is the Embolotherapy Market?
- What is the Embolotherapy Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




