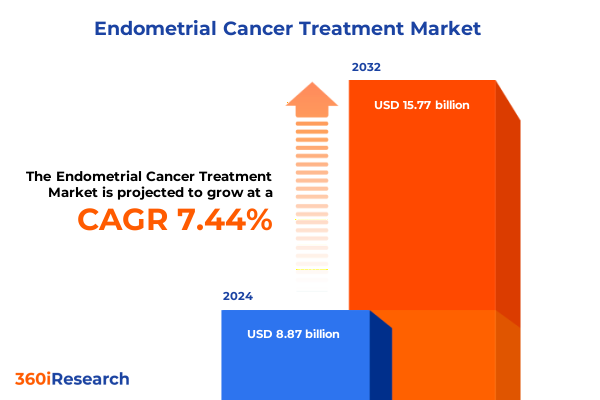
The Endometrial Cancer Treatment Market size was estimated at USD 9.38 billion in 2025 and expected to reach USD 9.93 billion in 2026, at a CAGR of 7.69% to reach USD 15.77 billion by 2032.
Endometrial Cancer Requires a Nuanced Therapeutic Approach as Incidence Rises and Clinical Complexity Demands Diversified Treatment Strategies
Endometrial cancer, the most prevalent type of uterine malignancy, arises from abnormal cell growth in the endometrial lining of the uterus and poses a growing public health challenge. According to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, an estimated 67,880 new cases and nearly 13,250 deaths from endometrial cancer will occur in the United States in 2024, reflecting a nearly 2% increase in mortality rates over recent years and underscoring the urgency for improved clinical interventions.
Epidemiological trends highlight a concerning rise in incidence across demographic groups, driven in part by increasing obesity rates, hormonal imbalances, and genetic predispositions. Analysis of data from 2013 through 2022 reveals that uterine cancer diagnoses climbed by approximately 0.7% annually, with age-adjusted death rates rising by 1.6% per year; projections indicate an even steeper trajectory among Black women, who face more aggressive tumor subtypes and delayed access to care.
Against this backdrop of rising disease burden and demographic disparities, the treatment paradigm has diversified beyond standard hysterectomy and adjuvant radiation. Clinicians now integrate systemic modalities-ranging from hormone therapy to advanced precision oncology and immunotherapy-to address both early-stage tumors and recurrent or metastatic disease, reflecting the critical need to tailor interventions to individual patient profiles.
Significant Advances in Endometrial Cancer Care Are Emerging from Immunotherapy, Precision Medicine, and Novel Combination Treatment Paradigms
Recent breakthroughs in immuno-oncology have reshaped the therapeutic landscape, offering new avenues to harness the patient’s own immune system against endometrial tumors. Checkpoint inhibitors such as pembrolizumab and dostarlimab now play pivotal roles in patients with mismatch repair–deficient or microsatellite instability–high cancers, with durable responses observed when these agents are combined with platinum-based chemotherapy or administered as monotherapies in refractory settings. Early-phase data further suggest that emerging agents targeting CTLA-4 or dual immune checkpoints may extend benefits to broader patient populations, marking a clear shift towards personalized immune-based regimens.
Parallel advances in targeted therapy capitalize on molecular vulnerabilities within the cancer cell. Inhibitors of mTOR, PARP, and tyrosine kinases have demonstrated meaningful activity, especially when deployed in combination with hormonal or antiangiogenic strategies. Novel agents such as ridaforolimus and temsirolimus continue to be evaluated, while FDA approvals of lenvatinib in combination with pembrolizumab have validated the strategy of combining targeted small molecules with immunotherapy to achieve synergistic antitumor effects and improved survival metrics in both MSI-H and non–MSI-H cohorts.
Surgical and radiotherapeutic approaches are also evolving to reduce morbidity and enhance precision. Minimally invasive techniques, including robot-assisted hysterectomy, are becoming standard in appropriate candidates, fostering faster recovery and reduced complication rates. Concurrently, MRI-guided radiation platforms are undergoing clinical evaluation to focus dose delivery on tumor volumes while sparing healthy tissues. These innovations, integrated with systemic therapies, support a multimodal framework that seeks to optimize local control and extend long-term survival in patients across all stages of disease.
Escalating Trade Policies Have Imposed Complex Tariff Burdens on Oncology Supply Chains Impacting Endometrial Cancer Treatment Accessibility
Heightened trade tensions and proposed tariff policies in 2025 have introduced new complexities for oncology supply chains, with direct implications for the accessibility and cost of endometrial cancer treatments. Johnson & Johnson projects a $400 million impact from existing worldwide tariffs-primarily on medical devices but also encompassing active pharmaceutical ingredients-underscoring the broader risks for manufacturers and healthcare providers alike. Industry surveys reveal that a majority of healthcare executives anticipate significant cost escalations for hospitals and payers, potentially translating into higher out‐of‐pocket expenses for patients and added pressure on reimbursement frameworks.
Particular concern has emerged around a newly imposed 245% tariff on Chinese‐sourced active pharmaceutical ingredients, a measure that threatens to disrupt supply reliability and incentivize shifts in manufacturing locations. Major pharmaceutical corporations are evaluating strategies to mitigate these exposures by diversifying sourcing to alternative markets such as India and Germany, though such transitions require rigorous quality assurance and could delay drug production timelines for critical oncology agents.
In response, several industry leaders have committed to reshoring investments and bolstering domestic production to hedge against tariff uncertainties. AstraZeneca announced a $50 billion US investment plan to expand R&D and manufacturing capacity across multiple states, a strategic recalibration aimed at preserving market access and demonstrating resilience amidst fluctuating trade policy.
Cross Segment Analysis Reveals Market Dynamics Across Treatment Modalities, Drug Classes, Administration Channels, and Care Settings in Endometrial Cancer
A comprehensive view of segmentation reveals that endometrial cancer treatment dynamics vary significantly by the type of therapy deployed. Chemotherapy retains a foundational role, often delivered as platinum-taxane regimens, while combination therapy-especially chemoimmunotherapy-has gained traction for its synergistic action. Hormonal therapy remains integral for low-grade, estrogen-driven tumors, with agents such as aromatase inhibitors and progestins offering disease control and quality-of-life benefits. Immunotherapy has emerged as a breakthrough segment, subdivided into CTLA-4 and PD-1 inhibitors, addressing both first-line and salvage therapy settings. Radiation therapy and surgery continue as cornerstone interventions, and targeted therapies-including mTOR, PARP, and tyrosine kinase inhibitors-offer precision treatment options aligned with specific molecular profiles.
Further stratification by line of therapy underscores the clinical imperative to sequence treatments effectively. First-line regimens prioritize maximal tumor cytoreduction and disease stabilization, while second- and third-line options increasingly rely on immuno-oncology and targeted agents to overcome resistance. In fourth-line and beyond scenarios, combination regimens and novel investigational drugs are deployed in refractory patient populations to extend survival where standard modalities fall short. Administration modes also shape patient experience, with oral agents facilitating outpatient management and intravenous infusions necessitating specialized infusion center infrastructure. Moreover, the real-world setting-ranging from ambulatory care clinics to hospital inpatient and outpatient environments-influences treatment accessibility, requiring tailored approaches to patient support and logistics coordination.
This comprehensive research report categorizes the Endometrial Cancer Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Drug Class
- Line Of Therapy
- Administration Setting
- Mode Of Administration
Regional Market Insights Highlight Variation in Treatment Adoption, Reimbursement Policies, and Access Challenges Across Americas, EMEA, and Asia-Pacific
In the Americas, the United States leads in early adoption of advanced immunotherapies and targeted treatment combinations, underpinned by rapid FDA approvals and robust clinical trial activity. The recent authorization of immunotherapy agents such as Imfinzi in combination with carboplatin and paclitaxel for mismatch repair–deficient endometrial cancer exemplifies this trend, driving increased integration of immune checkpoint inhibitors in both community oncology practices and academic centers.
Within Europe, Middle East, and Africa regions, treatment uptake is influenced by diverse reimbursement landscapes and regulatory frameworks. England’s NICE guidance endorsing dostarlimab with platinum-based chemotherapy for advanced or recurrent cases underscores alignment between clinical benefit and health technology assessment criteria. The NHS rollout via the Cancer Drugs Fund further demonstrates how managed access pathways can expedite patient entry to innovative therapies while additional evidence is generated.
Across the Asia-Pacific, countries such as Japan have rapidly embraced combination regimens, exemplified by the Ministry of Health’s approval of pembrolizumab plus lenvatinib for unresectable or recurrent endometrial carcinoma following progression on chemotherapy. This decision underscores regional regulatory collaboration and highlights the strategic importance of manufacturing partnerships to ensure consistent supply in markets with growing oncology needs.
This comprehensive research report examines key regions that drive the evolution of the Endometrial Cancer Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Competitive Landscape Overview Showcases Leading Biopharma Entities Driving Innovation in Endometrial Cancer Therapeutic Portfolios and Key Partnerships
GSK has significantly expanded its immuno-oncology footprint with a positive CHMP opinion and subsequent European Commission approval to broaden dostarlimab plus carboplatin-paclitaxel to all adult primary advanced or recurrent endometrial cancer patients, including mismatch repair–proficient and microsatellite-stable populations. This regulatory progression builds upon the RUBY phase III trial results, which demonstrated substantial overall survival benefits and established dostarlimab as the first immuno-oncology agent to significantly improve outcomes in this setting.
AstraZeneca’s Imfinzi and Merck’s pioneering lenvatinib-pembrolizumab combination further underscore competitive differentiation. The FDA approval of Imfinzi in DUO-E study populations and the expansion of pembrolizumab-lenvatinib into the Japanese market have diversified therapeutic arsenals and set new efficacy benchmarks. Meanwhile, Roche’s strategic moves to reduce intermediaries and bolster direct-to-patient supply chains position durvalumab as a potential disrupter in cost and access paradigms, reflecting a shifting balance between innovation and affordability in endometrial cancer care.
This comprehensive research report delivers an in-depth overview of the principal market players in the Endometrial Cancer Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- AstraZeneca PLC
- Bayer AG
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Bristol-Myers Squibb Company
- Celgene Corporation
- Context Therapeutics Inc.
- CooperSurgical Inc.
- Eisai Co., Ltd.
- Elekta AB
- F. Hoffmann-La Roche AG
- General Electric Company
- GlaxoSmithKline PLC
- Hologic Inc.
- Johnson & Johnson Services, Inc.
- Karyopharm Therapeutics Inc.
- Koninklijke Philips N.V.
- LiNA Medical ApS
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Siemens Healthineers AG
- Takeda Pharmaceutical Company Limited
Actionable Strategic Recommendations Encourage Investment in Innovation, Supply Chain Resilience, and Collaborations to Enhance Endometrial Cancer Care
Industry leaders should prioritize investment in localized manufacturing and supply chain diversification to mitigate tariff exposures and ensure uninterrupted delivery of critical oncology therapies; this includes establishing facilities in strategic regions and leveraging partnerships to secure alternative raw material sources. Concurrently, accelerating biomarker testing and precision treatment protocols will enhance patient stratification and optimize therapeutic outcomes, addressing both clinical needs and payer expectations. Finally, collaborative research initiatives between public agencies, academic institutions, and private stakeholders are imperative to sustain momentum in novel combination trials and to streamline regulatory pathways for emerging treatment modalities.
Comprehensive Research Methodology Employed Mixed Qualitative and Quantitative Approaches to Ensure Rigorous Analysis of Endometrial Cancer Treatment Trends
This research initiative integrated a comprehensive methodology combining quantitative data analysis with qualitative insights from key opinion leaders in oncology, clinical pharmacology, and health economics. Secondary data were gathered from peer-reviewed journals, regulatory databases, and proprietary clinical trial repositories. Primary research included in-depth interviews with oncologists and supply chain experts, along with validation workshops to refine analytical assumptions. The triangulation of these methods ensured a robust framework for discerning treatment trends, competitive dynamics, and policy impacts across multiple global markets.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Endometrial Cancer Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Endometrial Cancer Treatment Market, by Treatment Type
- Endometrial Cancer Treatment Market, by Drug Class
- Endometrial Cancer Treatment Market, by Line Of Therapy
- Endometrial Cancer Treatment Market, by Administration Setting
- Endometrial Cancer Treatment Market, by Mode Of Administration
- Endometrial Cancer Treatment Market, by Region
- Endometrial Cancer Treatment Market, by Group
- Endometrial Cancer Treatment Market, by Country
- United States Endometrial Cancer Treatment Market
- China Endometrial Cancer Treatment Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1590 ]
Concluding Insights Emphasize the Critical Interplay of Therapeutic Innovation, Policy Shifts, and Collaborations in Advancing Endometrial Cancer Care
In conclusion, the trajectory of endometrial cancer management reflects a confluence of immuno-oncology breakthroughs, targeted precision therapies, and refinements in surgical and radiotherapeutic interventions. Simultaneously, evolving trade policies underscore the necessity for resilient supply chains and strategic investments in domestic infrastructure. Segmentation analysis reveals nuanced market dynamics by therapy type, line of care, administration mode, and clinical setting, while regional insights highlight variation in adoption and access. Leading biopharma entities are competing to define the next generation of combination regimens, laying the groundwork for future innovation. These developments collectively chart a path toward more personalized, accessible, and effective treatment paradigms for patients worldwide.
Engage with Associate Director Ketan Rohom to Access the Comprehensive Market Research Report on Endometrial Cancer Treatment Innovations and Strategies
For a deeper exploration of emerging therapeutic opportunities, competitive dynamics, and strategic imperatives in endometrial cancer treatment, contact Ketan Rohom, the Associate Director of Sales & Marketing, to secure your organization’s access to the comprehensive, in-depth market research report.

- How big is the Endometrial Cancer Treatment Market?
- What is the Endometrial Cancer Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




