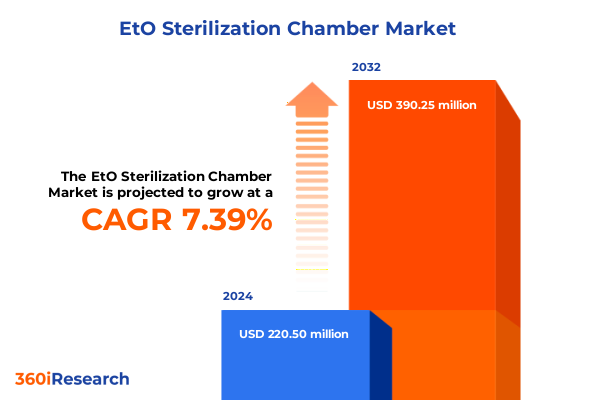
The EtO Sterilization Chamber Market size was estimated at USD 235.75 million in 2025 and expected to reach USD 253.75 million in 2026, at a CAGR of 7.46% to reach USD 390.25 million by 2032.
Unlocking the Critical Role of Ethylene Oxide Sterilization Chambers in Healthcare and Life Sciences Supply Chains Shaping Global Market Dynamics
Ethylene Oxide sterilization chambers serve as a cornerstone technology in the relentless pursuit of infection prevention across medical, pharmaceutical, and research domains. These systems leverage ethylene oxide gas to eradicate microbial contaminants on heat- and moisture-sensitive instruments, ensuring that critical devices meet stringent standards for patient safety. Driven by escalating expectations for sterilization efficacy, regulatory agencies worldwide have strengthened protocols, prompting end users to adopt high-performance chamber designs that deliver reliable, uniform sterilization cycles.
Against the backdrop of complex supply chains, evolving compliance requirements, and mounting sustainability concerns, stakeholders require a nuanced understanding of how Ethylene Oxide sterilization chambers integrate into broader healthcare and life sciences ecosystems. This introductory overview frames the essential attributes of these chambers-ranging from cycle parameters and aeration methods to safety controls and environmental considerations. By establishing foundational knowledge of operational principles and regulatory drivers, this section equips decision-makers with the context needed to navigate investment decisions, technology selection, and process optimization. Furthermore, it highlights the interdependence between sterilization infrastructure and overall facility throughput, underlining how chamber performance can directly influence clinical workflows, cost efficiencies, and long-term operational resilience.
Emerging Innovations and Disruptive Technologies Redefining Ethylene Oxide Sterilization Processes and Equipment Standards
Innovation is rapidly reshaping the paradigm of Ethylene Oxide sterilization, as manufacturers introduce digital controls, advanced cycle profiling, and next-generation sensor arrays that enhance both reliability and throughput. Modern chambers now integrate touchscreen interfaces, real-time monitoring dashboards, and automated data logging to facilitate seamless validation and regulatory compliance. Moreover, the adoption of vacuum-induced pre-conditioning and post-sterilization aeration sequences has reduced cycle times while ensuring more thorough gas penetration into complex device geometries.
In addition, intelligent connectivity is emerging as a transformative force: integrated IoT modules allow remote diagnostics and predictive maintenance, minimizing unplanned downtime and extending equipment lifecycles. Sustainability has also taken center stage, with new EO recapture systems and catalytic converters designed to mitigate environmental emissions. These eco-focused enhancements not only align with ever-tightening emissions regulations but also contribute to lower total cost of ownership by reclaiming excess gas. As a result, these converging innovations are redefining expectations for sterilization performance, setting a new standard for safety, efficiency, and environmental stewardship across diverse application areas.
Evaluating the Comprehensive Effects of 2025 United States Tariff Adjustments on Ethylene Oxide Sterilization Chamber Supply Chains
In 2025, several new tariff measures enacted by the United States have introduced additional costs on imported components and raw materials critical to Ethylene Oxide sterilization chamber production. These tariffs, targeting specific pressure vessel materials, electronic control modules, and specialty polymers, have reverberated throughout the supply chain, compelling manufacturers to reassess sourcing strategies and cost structures. Import duties of up to 10% on certain Chinese–manufactured sterilization controls have, in particular, eroded traditional cost advantages, driving some producers to repatriate assembly operations or qualify alternate suppliers in lower-cost regions.
Furthermore, the cumulative impact of these tariff adjustments extends beyond direct material expenses. Equipment OEMs have encountered increased logistics complexity, as multi-tiered duty classifications necessitate more rigorous classification audits and documentation. Compliance with customs requirements has become more labor intensive, prompting organizations to invest in trade compliance teams and automated tariff classification software. As a consequence, lead times have lengthened for bespoke chamber configurations, and upward pricing pressure has been observed across the board. To maintain competitiveness, many stakeholders are exploring nearshoring opportunities, leveraging free-trade agreements, and pursuing strategic partnerships to diversify their supplier base while ensuring uninterrupted access to critical subcomponents.
Unveiling Critical Segmentation Frameworks to Decode Market Dynamics Across Product Types, Techniques, Capacities, End Users, and Applications
Understanding market dynamics requires a detailed examination of product type, technique, chamber dimension, end-user profiles, and application areas. When observed through the lens of product type, benchtop chambers have gained traction in smaller clinics and research labs due to their compact footprints and lower upfront capital intensity, whereas floor-standing systems continue to dominate high-volume hospital central sterilization departments where capacity and throughput are paramount. Transitioning to technique segmentation reveals two primary cycle methodologies: gravity displacement cycles offer simplicity and cost-effectiveness for routine loads, while pre-vacuum cycles deliver enhanced penetration for complex instruments, supporting higher sterilization assurance levels.
Chamber size segmentation further elucidates distinct operational use cases. Below-400 liter chambers, subdivided into up to 200 liters and 200 to 400 liters, cater to point-of-use sterilization in decentralized settings, enabling rapid turnaround for emergency or outpatient procedures; mid-range units spanning 400 to 1000 liters, broken into 400 to 600 and 600 to 1000 liter classes, balance footprint considerations with moderate throughput demands; and larger solutions exceeding 1000 liters, categorized into 1000 to 2000 and above 2000 liter options, serve the most intensive needs of large hospital networks and contract sterilizers. Focusing on end-user segmentation reveals that hospitals, medical device manufacturers, pharmaceutical companies, and research institutes each impose unique requirements for validation, documentation, and cycle adaptability. Finally, application segmentation underscores how chambers are validated and configured for sterilizing food packaging substrates, delicate medical device assemblies, or active pharmaceutical ingredients, demonstrating versatile use across multiple verticals.
This comprehensive research report categorizes the EtO Sterilization Chamber market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Sterilization Technique
- Chamber Size
- End User
Mapping Regional Trends and Opportunities Spanning the Americas, Europe Middle East Africa, and Asia-Pacific Markets for Ethylene Oxide Sterilization
Regional landscapes exhibit marked disparities in regulatory frameworks, infrastructure maturity, and investment priorities. Within the Americas, particularly the United States and Canada, advanced healthcare systems drive demand for high-efficiency chambers with sophisticated data management and compliance features to satisfy FDA and Health Canada requirements. Latin American markets are gradually expanding capacity, with select national health initiatives accelerating procurement of modern sterilization platforms to strengthen infection control in public hospitals.
In Europe, Middle East, and Africa, stringent EU MDR regulations and ISO certification mandates have elevated baseline standards for equipment validation, prompting EU-based manufacturers to lead in cycle traceability and quality management. Concurrently, emerging markets in the Middle East and North Africa are investing in new hospital infrastructures and pharmaceutical clusters, generating opportunities for chamber providers that offer localized technical support and training services. Across sub-Saharan Africa, donor-funded programs aimed at reducing healthcare-associated infections are fostering incremental adoption of reliable sterilization systems.
Asia-Pacific presents a dual narrative of rapid industrial expansion and escalating end-user sophistication. China and India are not only significant producers of sterilization chambers but also burgeoning consumer markets for both entry-level and advanced pre-vacuum systems. Japan and South Korea, with their focus on automation and digital integration, are driving standards for smart sterilization, while Southeast Asian nations are investing in capacity upgrades to meet regional pharmaceutical manufacturing growth. These varied regional trajectories underscore the importance of nuanced go-to-market strategies that align product portfolios with localized regulatory expectations, service capabilities, and capital expenditure cycles.
This comprehensive research report examines key regions that drive the evolution of the EtO Sterilization Chamber market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Players Driving Innovation Strategic Partnerships and Competitive Differentiation in Ethylene Oxide Sterilization Equipment
A handful of global and regional players are shaping the competitive landscape through continuous product innovation, strategic alliances, and geographic expansion. Leading OEMs have broadened their portfolios to include modular chamber architectures that support scalable deployment across diverse facility sizes and application requirements. Several firms have forged partnerships with chemical suppliers to co-develop EO recapture technologies, reinforcing commitments to sustainability and regulatory compliance.
Meanwhile, acquisition activity has intensified as market leaders seek to augment service networks and digital offerings. Corporations with well-established after-sales infrastructures are leveraging that advantage by incorporating remote monitoring services and artificial intelligence-enabled diagnostics, elevating customer value propositions beyond traditional equipment sales. In parallel, select niche specialists are capitalizing on unmet needs in emerging markets, securing local certifications and establishing regional manufacturing footprints to expedite delivery and tailor system configurations. This dynamic interplay of R&D investment, M&A pursuits, and regional alliances ensures that the competitive hierarchy remains fluid, rewarding companies that can anticipate end-user trends and swiftly adapt their product and service models.
This comprehensive research report delivers an in-depth overview of the principal market players in the EtO Sterilization Chamber market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Adinath International
- Alliance Elemech
- Henan Huatai Cereals And Oils Machinery Co. Ltd.
- Riya Engineering
- Salvesion Pharm
- Star Techno Engineering Private Limited
- Steri Healthcare Systems
- Steri-Techno Fab
- Sterimac India
- Sun Sterifaab Pvt. Ltd.
Strategic Imperatives and Best Practices for Industry Leaders to Enhance Efficiency Sustainability and Compliance in Sterilization Operations
To thrive amid intensifying cost pressures, regulatory scrutiny, and evolving technology demands, industry leaders must adopt a series of targeted initiatives. First, integrating advanced sensor technologies and machine-learning-driven diagnostics can preempt system failures and optimize cycle performance, reducing unplanned downtime and maintenance overhead. In addition, diversifying supply chains by qualifying alternate component suppliers and exploring nearshoring options will mitigate the risk of tariff-induced cost escalation while shortening lead times for critical spare parts.
Furthermore, committing to environmental stewardship through adoption of EO gas recapture and catalytic destruction systems not only fulfills tightening emissions regulations but also enhances corporate sustainability profiles. Companies should also invest in modular chamber designs that enable seamless scalability, allowing customers to align capital expenditure with actual throughput requirements. Strengthening after-sales service capabilities-particularly remote monitoring, predictive maintenance contracts, and virtual training platforms-will foster deeper customer engagement and recurring revenue streams. Lastly, collaborating with regulatory bodies and standards organizations to shape emerging guidelines can secure early mover advantages and streamline market access for next-generation sterilization solutions.
Outlining Rigorous Research Methodology Integrating Primary Interviews Secondary Analysis and Data Triangulation to Ensure Comprehensive Insights
This analysis is grounded in a robust methodology that combines both primary and secondary research techniques. In the primary phase, expert interviews were conducted with equipment OEM executives, regulatory authorities, end-user sterilization managers, and chemical suppliers to capture firsthand insights on technological innovations, compliance challenges, and procurement strategies. Data from these discussions was synthesized with secondary sources, including peer-reviewed journals, technical standards documentation, patent filings, and industry white papers, to ensure both depth and rigor.
Quantitative data points were triangulated across multiple channels-financial reports, customs databases, and installation records-to validate market movements and tariff impacts. A structured framework guided the segmentation analysis, ensuring consistency across product types, techniques, chamber capacities, end-user verticals, and application domains. Furthermore, regional findings were corroborated through local distributor feedback and field surveys in key markets. Finally, all insights underwent an internal validation workshop with cross-functional experts to refine conclusions and recommendations, guaranteeing that the report reflects a comprehensive, evidence-based perspective on Ethylene Oxide sterilization chamber dynamics.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our EtO Sterilization Chamber market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- EtO Sterilization Chamber Market, by Product Type
- EtO Sterilization Chamber Market, by Sterilization Technique
- EtO Sterilization Chamber Market, by Chamber Size
- EtO Sterilization Chamber Market, by End User
- EtO Sterilization Chamber Market, by Region
- EtO Sterilization Chamber Market, by Group
- EtO Sterilization Chamber Market, by Country
- United States EtO Sterilization Chamber Market
- China EtO Sterilization Chamber Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1431 ]
Consolidating Key Findings and Future Outlook to Empower Stakeholders with Actionable Knowledge on Ethylene Oxide Sterilization Market Dynamics
In closing, the Ethylene Oxide sterilization chamber arena is experiencing accelerated evolution driven by technological innovation, heightened regulatory requirements, and shifting trade policies. Breakthroughs in digital monitoring, vacuum cycle optimization, and environmental controls are redefining performance benchmarks, while new tariff structures have prompted a strategic realignment of supply chains and sourcing approaches. Detailed segmentation highlights the diverse needs of hospitals, medical device manufacturers, pharmaceutical producers, and research institutions, each demanding customized solutions that balance capacity, cycle efficacy, and cost efficiency.
Regionally, advanced healthcare markets in the Americas and Europe demand sophisticated data integration and compliance features, whereas emerging economies in the Middle East, Africa, and Asia-Pacific present opportunities for growth through infrastructure investments and localized manufacturing initiatives. Competitive dynamics continue to pivot on R&D prowess, strategic alliances, and after-sales service excellence. By adhering to recommended best practices-ranging from modular design adoption to proactive environmental compliance-industry participants can not only navigate current challenges but also position themselves for sustained leadership in an increasingly complex landscape. This executive summary underscores the imperative for organizations to leverage data-driven strategies and cross-functional collaboration in order to capture emerging opportunities and drive long-term resilience.
Engage with the Associate Director Sales & Marketing to Secure Your Comprehensive Ethylene Oxide Sterilization Chamber Market Research Report
To explore the full depth of market drivers, competitive landscapes, and strategic imperatives within the Ethylene Oxide sterilization chamber segment, we invite you to connect with Ketan Rohom, Associate Director of Sales & Marketing. His expertise and understanding of industry challenges will guide you through tailored insights that align with your organization’s objectives. Gain early access to detailed analyses, proprietary data models, and forward-looking scenarios that enable you to seize emerging opportunities and mitigate supply chain disruptions. Engage with Ketan to secure your comprehensive Ethylene Oxide Sterilization Chamber Market Research Report and empower your strategic planning with evidence-based recommendations.

- How big is the EtO Sterilization Chamber Market?
- What is the EtO Sterilization Chamber Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




