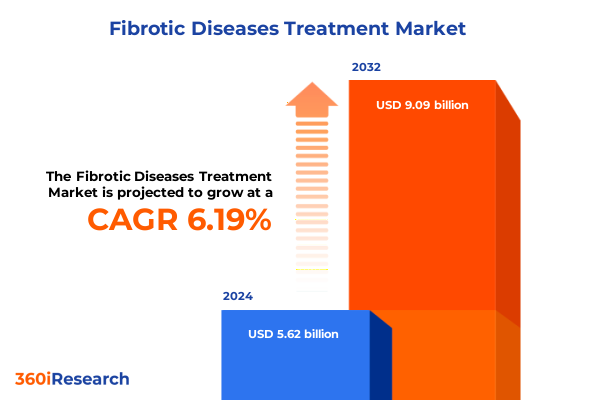
The Fibrotic Diseases Treatment Market size was estimated at USD 5.62 billion in 2024 and expected to reach USD 5.95 billion in 2025, at a CAGR of 6.19% to reach USD 9.09 billion by 2032.
Pioneering Breakthroughs and Emerging Therapeutic Avenues Define the Modern Fibrotic Diseases Treatment Ecosystem with Unprecedented Momentum
The landscape of fibrotic diseases treatment has entered a new era characterized by scientific breakthroughs and an intensified focus on patient-centric care pathways. Historically constrained by a limited arsenal of palliative measures, the therapeutic environment is now witnessing a convergence of cutting-edge research disciplines-from molecular biology and immunology to advanced imaging techniques-that collectively paint a more nuanced portrait of fibrosis pathogenesis. As a result, stakeholders across academia, industry, and clinical practice are coalescing around emerging modalities designed to target fibrotic processes at the cellular and molecular levels.
Within this dynamic context, the introduction of novel small molecule inhibitors, biologics, and cell-based therapies is redefining clinical protocols and patient outcomes. These innovations are bolstered by advancements in diagnostic precision, including biomarker discovery and non-invasive monitoring technologies, which enable earlier intervention and real-time assessment of therapeutic effectiveness. Simultaneously, regulatory agencies are adapting frameworks to expedite the evaluation of therapies that address significant unmet needs, thereby shortening the timeline from bench to bedside.
Against this backdrop, it is essential for decision-makers to appreciate not only the scientific underpinnings of these developments but also the structural shifts in clinical trial design, stakeholder collaboration, and reimbursement models. This introductory analysis lays the groundwork for understanding how the fibrotic diseases arena is evolving and sets the stage for a deeper examination of transformative shifts, trade dynamics, segmentation insights, and strategic imperatives in the sections that follow.
Fundamental Therapeutic Paradigms Shift as Innovative Modalities Reshape Clinical Approaches and Patient Outcomes in Fibrotic Diseases Treatment
The therapeutic paradigm for fibrotic diseases has undergone seismic shifts in recent years, driven by a deeper understanding of the molecular and cellular drivers of fibrosis. Researchers have moved beyond generalized anti-inflammatory approaches to develop highly specific inhibitors of pro-fibrotic signaling pathways, such as TGF-β and PDGF cascades. In parallel, the field has embraced regenerative medicine, leveraging mesenchymal stem cells and induced pluripotent stem cell platforms to promote tissue repair where fibrotic scarring once proved irreversible.
Moreover, the integration of gene-editing technologies like CRISPR and RNA interference strategies is opening doors to permanent modulation of genes implicated in fibrotic progression. This marks a fundamental shift from symptomatic relief toward curative intent. Complementing these advances is the deployment of artificial intelligence and machine learning algorithms, which are revolutionizing patient stratification, optimizing trial cohort selection, and predicting therapeutic responders through the analysis of multidimensional datasets.
These transformative shifts extend to peripheral domains as well: companion diagnostics now provide tailored insights into patient-specific fibrotic phenotypes, while digital health tools monitor adherence and physiological parameters outside of clinical settings. Such innovations are not only enhancing clinical outcomes but also reshaping the competitive landscape by blurring lines between pharmaceutical, biotech, and medtech enterprises. Collectively, these advances illustrate how the fibrotic diseases treatment environment is evolving into an interdisciplinary, data-driven ecosystem focused on precision interventions.
Comprehensive Assessment of 2025 United States Tariff Implications on Supply Chains Clinical Trials and Therapeutic Accessibility for Fibrotic Diseases
The introduction of new tariffs by the United States in 2025 has exerted multifaceted pressures across the supply chains underpinning fibrotic diseases treatment development. Key raw materials, such as specialized polymers used in formulation processes and active pharmaceutical ingredients sourced internationally, have encountered elevated import duties. This escalation in input costs has, in turn, influenced contract manufacturing organizations, prompting renegotiations of pricing structures and supply agreements.
Complicating matters further, tariffs on medical device components-ranging from inhalation delivery mechanisms to intravenous infusion pumps-have driven procurement teams to seek alternative vendors or innovate with domestic fabrication capabilities. While these efforts mitigate immediate cost hikes, they also introduce potential bottlenecks in quality assurance and regulatory compliance, as manufacturers adapt to new standards and inspection protocols.
Clinical trial operations have not been immune to these dynamics. An uptick in logistics expenses, combined with tightened budgets, has led sponsors to reevaluate the geographic distribution of trial sites and favor centralized laboratory services. In some instances, trial timelines have been extended to accommodate customs clearance delays and supply chain recalibrations. Despite these challenges, the industry has responded with strategic measures, including vertical integration of supply networks and the cultivation of tariff-exempt trade zones, to preserve the continuity of therapy development and safeguard patient access.
Strategic Segmentation Perspectives Illuminate Distinct Treatment Modalities Patient Profiles and Care Pathways Across the Fibrotic Diseases Spectrum
A nuanced examination of fibrotic diseases treatment reveals that therapeutic approaches diverge substantially across multiple dimensions. Patients with cardiac fibrosis require interventions that address both myocardial scarring and hemodynamic compromise, whereas those with liver fibrosis often rely on agents that modulate hepatic stellate cell activation in concert with lifestyle interventions. Pulmonary fibrosis management has evolved to include targeted inhalation therapies alongside systemic immune-modulating drugs, and renal fibrosis protocols frequently integrate antifibrotic medications with dialysis and transplantation modalities.
Treatment modalities themselves span traditional pharmaceuticals, organ transplantation, and supportive oxygen therapy, with each category offering distinct clinical benefits and logistical considerations. Administration techniques further differentiate therapies; inhalation routes optimize pulmonary drug delivery, injection and intravenous methods ensure bioavailability in systemic circulation, oral formulations prioritize patient convenience, and topical applications offer localized effect for organ-adjacent interventions.
Timing of intervention is equally critical. Early-stage fibrosis benefits from preventative treatment strategies designed to interrupt pro-fibrotic signaling before extensive extracellular matrix deposition occurs. Advanced-stage fibrosis often necessitates combination regimens that target both symptom control and scar tissue regression, while end-stage organ failure may demand life-saving transplantation and supportive care. Finally, preventative paradigms targeting pre-fibrosis populations focus on risk factor mitigation and surveillance.
Across end users, the adoption of these therapies varies significantly. Academic and research institutes play a pivotal role in driving experimental protocols and translational studies, hospitals serve as hubs for complex therapeutic regimens and post-transplant care, and specialty clinics offer niche expertise that accelerates patient screening and follow-up. Together, these segments underscore the importance of tailored strategies that account for disease subtype, therapeutic modality, administration route, disease stage, and care setting.
This comprehensive research report categorizes the Fibrotic Diseases Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Disease Type
- Treatment Type
- Route of Administration
- Digonosis
- Stage of Disease
- End User
Regional Dynamics and Healthcare Infrastructure Variations Shape the Adoption of Innovative Fibrotic Diseases Treatments Across Global Markets
Geographic regions display pronounced disparities in healthcare infrastructure, regulatory frameworks, and patient demographics, each shaping the trajectory of fibrotic diseases treatment in unique ways. In the Americas, robust clinical trial networks and well-established reimbursement systems facilitate rapid adoption of breakthrough therapies, while a surge in epidemiological studies drives awareness of regional disease prevalence and risk factors.
Over in Europe, the Middle East, and Africa, regulatory harmonization efforts and public-private partnerships are unlocking access to novel therapeutics, though variability in healthcare spending and infrastructure can influence rollout schedules. Collaborative platforms among academic centers and regional health authorities are increasingly employed to streamline approval processes and address disparities in patient care.
The Asia-Pacific corridor represents another vital dimension, combining large patient populations with cost-sensitive markets and emerging biotech ecosystems. Regulatory bodies across this region are fostering innovation through pilot programs that expedite conditional approvals for therapies tackling high-burden diseases. Concurrently, local manufacturing capabilities and government-backed health initiatives are reducing dependency on imports, thereby supporting sustainable long-term growth.
Transitioning between these territories requires companies to adapt commercialization strategies, regulatory dossiers, and stakeholder engagement models. Recognizing regional nuances in clinical practice patterns, payer policies, and patient access pathways is essential for stakeholders seeking to maximize the impact of emerging fibrotic disease therapies on a global scale.
This comprehensive research report examines key regions that drive the evolution of the Fibrotic Diseases Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Competitive Landscape Highlighting Leading Biopharmaceutical Innovators Driving Next-Generation Therapies in Fibrotic Diseases Treatment and Collaboration Trends
The competitive arena of fibrotic diseases treatment is defined by a handful of biopharmaceutical leaders and nimble specialty players driving the next wave of innovation. Large-cap organizations have leveraged expansive R&D budgets to develop first-in-class small molecules and biologics targeting core fibrotic pathways. These companies often pursue strategic alliances with academic institutions to access cutting-edge discoveries and share the burden of early-stage research costs.
At the same time, mid-size and emerging biotech firms are making significant strides by focusing on niche indications or employing platform technologies such as gene therapy vectors and cell-based regeneration approaches. Collaboration among these entities and contract research organizations has become increasingly commonplace, enabling rapid iteration of clinical protocols and flexible scaling of manufacturing capacities.
Partnerships between pharmaceutical incumbents and device manufacturers are also on the rise, spurred by the need for integrated solutions that combine therapeutic compounds with specialized delivery systems. Licensing agreements, joint ventures, and co-development pacts illustrate the industry’s recognition that interdisciplinary efforts accelerate the translation of laboratory breakthroughs into approved therapies. As these collaborative models continue to evolve, they are setting new benchmarks for speed-to-market and patient engagement initiatives.
This comprehensive research report delivers an in-depth overview of the principal market players in the Fibrotic Diseases Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- CHIESI Farmaceutici S.p.A.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- Galapagos NV
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Intercept Pharmaceuticals, Inc. by Alfasigma Group
- Ipsen Pharma
- Kyorin Pharmaceutical Co., Ltd.
- Merck & Co., Inc.
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Redx Pharma Plc
- Regeneron Pharmaceuticals, Inc.
- Sanofi S.A.
- Santen Pharmaceutical Co., Ltd.
- Takeda Pharmaceutical Company Limited
- Tanabe Pharma Corporation
- Teva Pharmaceutical Industries Ltd.
- Vertex Pharmaceuticals Incorporated
Strategic Imperatives and Tactical Roadmap for Industry Leaders to Accelerate Innovation Streamline Development Pathways and Enhance Patient-Centric Outcomes
To capitalize on the momentum in fibrotic diseases treatment, industry leaders should prioritize the establishment of integrated diagnostic platforms that combine imaging, liquid biopsy, and molecular profiling to identify patients most likely to benefit from targeted interventions. By investing in digital health infrastructures and real-world data capture, stakeholders can generate compelling evidence to support reimbursement negotiations and post-marketing surveillance.
Supply chain resilience must also remain a top priority. Diversifying sourcing strategies for critical raw materials and device components, while exploring local manufacturing partnerships, will mitigate tariff-related disruptions and strengthen business continuity. Concurrently, adaptive clinical trial designs-incorporating decentralized models, interim analyses, and biomarker-driven cohorts-can reduce operational risk and accelerate regulatory submissions.
Finally, forging collaborative ecosystems that bring together academic centers, patient advocacy groups, and payers will enhance stakeholder alignment and ensure patient needs remain at the forefront of product development. Organizations that embrace open innovation, co-development agreements, and cross-sector consortia will be best positioned to navigate the complex regulatory environment and deliver meaningful therapeutic advances for patients living with fibrotic diseases.
Comprehensive Research Approach Integrating Quantitative Clinical Data Analysis with Qualitative Expertise from Opinion Leaders and Field Practitioners
This analysis is grounded in a rigorous research approach that integrates quantitative assessments of clinical trial registries, peer-reviewed literature, and treatment utilization databases with qualitative insights gathered from in-depth interviews. Expert perspectives were solicited from leading fibrosis researchers, practicing clinicians, and regulatory advisors, offering a multidimensional understanding of therapeutic efficacy, safety profiles, and development challenges.
Primary data collection included structured conversations with opinion leaders to explore emerging scientific hypotheses and real-world clinical experiences. Simultaneously, secondary research encompassed systematic reviews of recent publications, guideline updates, and regulatory advisory announcements. Comparative evaluations of administration routes, treatment schedules, and care pathways were performed to contextualize product positioning across different stages of disease progression.
Throughout the methodology, special attention was given to ensuring data integrity and minimizing bias. Cross-validation techniques were employed to triangulate findings, while peer review from external experts provided an additional layer of critical scrutiny. This comprehensive framework supports robust, actionable insights designed to inform strategic decision-making and drive the next generation of fibrotic diseases therapies.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Fibrotic Diseases Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Fibrotic Diseases Treatment Market, by Disease Type
- Fibrotic Diseases Treatment Market, by Treatment Type
- Fibrotic Diseases Treatment Market, by Route of Administration
- Fibrotic Diseases Treatment Market, by Digonosis
- Fibrotic Diseases Treatment Market, by Stage of Disease
- Fibrotic Diseases Treatment Market, by End User
- Fibrotic Diseases Treatment Market, by Region
- Fibrotic Diseases Treatment Market, by Group
- Fibrotic Diseases Treatment Market, by Country
- United States Fibrotic Diseases Treatment Market
- China Fibrotic Diseases Treatment Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1431 ]
Integrated Synthesis of Emerging Therapeutic Opportunities Challenges and Strategic Pathways Guiding the Future of Fibrotic Diseases Treatment Innovation
The cumulative findings underscore a pivotal moment in fibrotic diseases treatment, where scientific innovation, collaborative models, and strategic resilience converge to redefine patient care. Breakthroughs in targeted therapies and regenerative approaches offer new hope for halting or reversing fibrosis across multiple organs. At the same time, evolving regulatory environments and shifting trade dynamics emphasize the need for agility in supply chain management and stakeholder engagement.
Segmentation analysis demonstrates that tailoring interventions by disease subtype, treatment modality, administration method, disease stage, and end-user setting is critical to maximizing clinical effectiveness and operational efficiency. Regional insights reinforce the importance of adapting market entry and commercialization strategies to the unique regulatory landscapes, healthcare infrastructures, and patient demographics of the Americas, Europe, Middle East & Africa, and Asia-Pacific.
Leading companies are already capitalizing on these opportunities through strategic alliances, platform technologies, and innovative trial designs. Looking ahead, organizations that commit to integrated diagnostics, diversified supply networks, and open innovation partnerships will be best equipped to navigate the complexities of the fibrotic disease landscape. This integrated synthesis provides a clear roadmap for stakeholders seeking to deliver transformative therapies and improve outcomes for patients worldwide.
Connect with Our Associate Director to Secure Strategic Insights and Comprehensive Analysis for Accelerating Growth in Fibrotic Diseases Treatment Markets
For tailored insights and strategic intelligence that can propel your organization’s initiatives in fibrotic diseases treatment forward, connect directly with Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch. Ketan brings a wealth of industry expertise and can guide you through the comprehensive research findings, helping you align your development strategies with the latest therapeutic innovations and regulatory considerations.
Engaging with Ketan ensures that you have personalized access to deep-dive analyses, customized data summaries, and expert recommendations designed to meet your unique business challenges. Reach out to explore flexible engagement models, receive exclusive executive briefings, and secure early access to forthcoming white papers and thought-leadership reports.
Invest in knowledge that empowers you to stay ahead in a rapidly evolving landscape-schedule a consultation with Ketan Rohom today to translate research insights into actionable growth strategies for your fibrotic diseases treatment programs.

- How big is the Fibrotic Diseases Treatment Market?
- What is the Fibrotic Diseases Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




