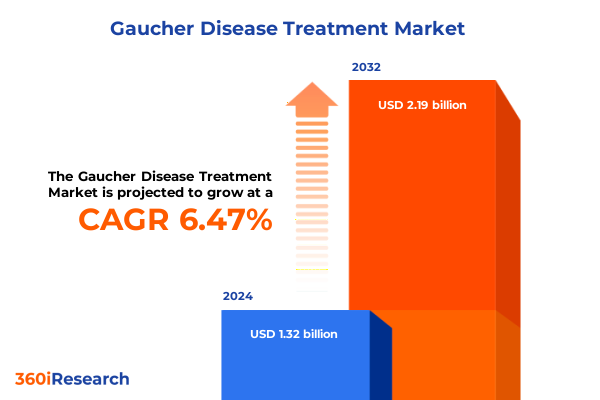
The Gaucher Disease Treatment Market size was estimated at USD 1.40 billion in 2025 and expected to reach USD 1.47 billion in 2026, at a CAGR of 6.63% to reach USD 2.19 billion by 2032.
Unveiling the Dynamic Evolution of Gaucher Disease Therapy Landscape Through Treatment Innovations and Patient-Centric Care Advances
The landscape of Gaucher disease treatment has undergone profound transformation over recent years, fueled by relentless scientific innovation and an unwavering commitment to patient welfare. This inherited lysosomal storage disorder demands nuanced approaches that extend beyond conventional enzyme replacement therapies. Throughout this evolution, stakeholders have confronted challenges pertaining to treatment accessibility, adherence, and long-term disease management. As the field matures, the interplay between novel modalities, regulatory rigor, and patient-centric care models becomes increasingly vital.
Amid these developments, the therapeutic toolkit has expanded to encompass targeted small molecules, biologics, and supportive therapies designed to mitigate disease progression while enhancing quality of life. Healthcare providers are adapting protocols to integrate both traditional infusions and oral regimens, while payers and policymakers grapple with the economic implications of lifelong treatments. In parallel, patient advocacy groups and industry consortia have intensified efforts to raise awareness, streamline diagnostic pathways, and secure favorable reimbursement frameworks. Together, these forces define the current phase of Gaucher disease treatment: one marked by collaborative innovation and a relentless pursuit of optimized patient outcomes.
Navigating the Paradigm Shifts in Gaucher Disease Treatment: How Novel Modalities and Strategic Collaborations Are Redefining Care Pathways
Over the past decade, Gaucher disease treatment has shifted from a singular reliance on biweekly infusions toward a diversified portfolio of targeted strategies that emphasize convenience, specificity, and durability. Enzyme replacement therapies retain a foundational role, but their dominance has been complemented by the rise of substrate reduction and pharmacological chaperone approaches. Emerging investigational modalities, including gene editing and RNA interference, further exemplify the field’s commitment to transformative outcomes.
Strategic collaborations between biotechnology firms, academic institutions, and patient advocacy coalitions have accelerated clinical development and expanded access initiatives. Regulatory authorities have demonstrated greater flexibility in orphan drug pathways, enabling faster approvals while upholding stringent safety standards. This environment has fostered the rapid adoption of oral therapies, which alleviate infusion burdens and improve adherence. As real-world evidence programs proliferate, clinical decision makers gain deeper insights into long-term efficacy and safety across diverse patient cohorts, facilitating more nuanced care pathways.
Consequently, industry participants are reevaluating traditional market approaches, placing greater emphasis on integrated care models that encompass telemedicine, home-based administration support, and remote monitoring. These shifts herald a new era in which therapeutic innovation is intimately linked with patient experience, driving sustained advances in Gaucher disease management.
Examining the Intensifying Effects of 2025 United States Tariffs on Gaucher Disease Therapeutic Supply Chains, Pricing Dynamics, and Access
In 2025, the United States government implemented a targeted tariff structure affecting imported biologic therapies, including those vital for Gaucher disease management. These levies have exacerbated logistical complexities within the supply chain, compelling manufacturers to reevaluate global distribution strategies and manage raw material sourcing under tighter cost constraints. Providers and treatment centers are now navigating increased procurement expenses, which ripple through pricing agreements and reimbursement negotiations.
Patients dependent on enzyme replacement infusions have encountered variable lead times and potential treatment delays as distributors adapt to higher import duties. The resultant unpredictability has intensified calls for localized manufacturing alternatives and greater supply chain transparency. Payers, meanwhile, are scrutinizing formulary compositions and adjusting coverage policies to accommodate altered cost structures, a move that may influence therapy selection and patient access in the short term.
Amid these challenges, biopharmaceutical companies are accelerating investments in domestic production capabilities and exploring hedging strategies to mitigate tariff-driven price volatility. Collaborative initiatives with contract manufacturing organizations aim to minimize disruptions and secure uninterrupted therapy availability. While these adaptations require significant upfront capital, they underscore a strategic pivot toward supply chain resilience that promises to safeguard patient care against future trade policy fluctuations.
Uncovering Strategic Segmentation in Gaucher Disease Treatment by Therapy Modality, Disease Variant, Administration Route, User Setting, and Patient Profile
A nuanced understanding of treatment segmentation reveals pivotal insights for optimally positioning therapies within the Gaucher disease landscape. When examining treatment types, enzyme replacement therapies such as Imiglucerase, Taliglucerase Alfa, and Velaglucerase Alfa continue to anchor clinical practice, benefiting from robust efficacy profiles and established reimbursement frameworks, whereas substrate reduction therapies including Eliglustat and Miglustat are gaining traction by catering to maintenance regimens and offering oral convenience. Chaperone therapy, notably Ambroxol, remains in early adoption phases, with ongoing studies exploring its potential to enhance residual enzyme function.
Disease type segmentation underscores the distinct management requirements for Type 1 patients, who constitute the majority cohort and often transition to oral regimens, contrasted with Types 2 and 3, which demand aggressive neurological monitoring and multidisciplinary care. Administration mode further differentiates treatment dynamics: intravenous infusions predominantly delivered in specialized clinics or hospital settings have given way to expanded oral administration, enabling home-based care and reducing the logistical burden on both patients and healthcare facilities.
Analyzing end-user contexts paints a detailed picture of care delivery pathways. General and specialty clinics have historically hosted most infusions, yet nurse-administered and self-administered home care models are swiftly scaling to meet patient demand for convenience and personalized support. Inpatient scenarios within private and public hospitals continue to play a critical role for severe presentations and complex cases. Finally, patient group distinctions between adult and pediatric cohorts highlight divergent needs for dose titration, long-term monitoring, and psychosocial support, reaffirming the necessity for tailored intervention strategies across age spectrums.
This comprehensive research report categorizes the Gaucher Disease Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Disease Type
- Administration Mode
- End User
- Patient Group
Exploring Regional Dynamics Shaping Gaucher Disease Care in the Americas, Europe Middle East & Africa, and Asia-Pacific Patient Treatment Ecosystems
Regional nuances exert a profound influence on Gaucher disease treatment access and adoption across global markets. In the Americas, North America remains the epicenter of innovation, driven by well-established regulatory frameworks, comprehensive insurance coverage models, and a mature biopharmaceutical ecosystem. Treatment adoption benefits from strong patient advocacy networks and high physician awareness, while Latin American markets demonstrate growing interest in oral therapies, albeit constrained by varied reimbursement landscapes and infrastructure gaps.
Within Europe, Middle East & Africa, Western European countries set the benchmark for timely approvals and favorable pricing negotiations, supported by parallel trade and cross-border healthcare initiatives. By contrast, markets in the Middle East and Africa exhibit significant heterogeneity in diagnostic capabilities and reimbursement policies, prompting multinational stakeholders to deploy tiered access programs and capacity building efforts. Private hospitals often bridge critical care gaps, while public healthcare systems manage broader patient populations under more stringent budgetary constraints.
Asia-Pacific presents a tapestry of emerging and established markets. In Japan and Australia, robust regulatory alignment and patient support schemes facilitate rapid uptake of novel therapies. Meanwhile, China’s inclusion of key treatments in the National Reimbursement Drug List has catalyzed local adoption, even as procedural complexities persist. Southeast Asian and Pacific Island nations are gradually enhancing rare disease governance, with regional partnerships and digital health interventions accelerating treatment awareness and delivery across diverse healthcare infrastructures.
This comprehensive research report examines key regions that drive the evolution of the Gaucher Disease Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Illuminating Competitive Strategies of Leading Gaucher Disease Treatment Innovators Driving Pipeline Expansion, Partnerships, and Market Positioning
Leading industry participants have cemented their positions in the Gaucher disease arena through strategic portfolio expansions, collaborative research endeavors, and targeted lifecycle management initiatives. Sanofi Genzyme continues to refine its enzyme replacement offerings while advancing clinical investigations into next-generation small molecules and chaperone combinations to broaden therapeutic choice and address unmet neurological indications. Pfizer has leveraged its bioproduction expertise to optimize Taliglucerase Alfa manufacturing, entering capacity-sharing agreements that bolster supply reliability and support slower-growing markets.
Takeda’s integration of Velaglucerase Alfa has yielded operational synergies, with cross-divisional pipelines exploring innovative delivery systems and combination regimens. Actelion’s Miglustat program underscores the value of oral substrate reduction in maintenance therapy, with ongoing real-world studies designed to delineate long-term safety profiles and patient-reported outcomes. Concurrently, select biotechnology firms are charting new territory by advancing gene therapy candidates through early-phase trials, reflecting a shared ambition to pursue durable, potentially curative interventions.
Collectively, these competitive strategies reflect an ecosystem in which established leaders and emerging players vie to enhance differentiation through clinical innovation, market access initiatives, and patient support platforms. Such dynamics continue to drive incremental progress while setting the stage for potential paradigm shifts in treatment standards.
This comprehensive research report delivers an in-depth overview of the principal market players in the Gaucher Disease Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Amicus Therapeutics, Inc.
- AVROBIO, Inc.
- CANbridge Life Sciences Ltd.
- CHIESI Farmaceutici S.p.A.
- Dr. Reddy’s Laboratories Ltd.
- Evotec SE
- Freeline Therapeutics Limited
- Gain Therapeutics, Inc.
- GSK PLC
- ISU ABXIS
- JCR Pharmaceuticals Co., Ltd.
- Lysogene
- Merck & Co., Inc.
- Pfizer Inc.
- Prevail Therapeutics by Eli Lilly and Company
- Protalix
- Sanofi S.A.
- Shire PLC by Takeda Pharmaceutical Company Limited
- Takeda Pharmaceutical Company Limited
Crafting Practical Strategies for Industry Stakeholders to Enhance Patient Outcomes, Optimize Treatment Adoption, and Navigate Regulatory Hurdles
Industry stakeholders poised for success will prioritize multifaceted strategies that reinforce patient outcomes, streamline treatment adoption, and navigate evolving regulatory environments. First, expanding patient support and education programs is essential to fostering adherence, particularly as oral administration gains prominence. Deploying digital platforms for treatment reminders, virtual consultations, and side-effect monitoring can significantly enhance the patient experience while alleviating provider workloads.
Second, building supply chain resilience through diversified manufacturing footprints and strategic partnerships with contract organizations will mitigate the impact of tariff fluctuations and distribution bottlenecks. Early engagement with payers to negotiate value-based agreements and secure formulary inclusion is imperative, particularly as cost pressures intensify under shifting trade policies. Additionally, investing in real-world evidence generation will inform payer discussions, substantiate long-term efficacy, and differentiate offerings in competitive tenders.
Lastly, forging collaborative alliances with specialist clinics, patient advocacy groups, and telehealth providers can expand treatment reach into underserved populations and emerging markets. By aligning commercial strategies with holistic care pathways that encompass neurological assessment, psychosocial support, and multidisciplinary coordination, organizations can enhance therapeutic impact and build enduring stakeholder trust.
Detailing the Rigorous Research Methodology Employed to Ensure Robust Data Integrity, Expert Validation, and Comprehensive Insight Generation
The foundation of this analysis rests on a dual-pronged research approach combining rigorous secondary and primary investigations. Initially, a thorough review of peer-reviewed literature, regulatory submissions, clinical trial registries, and pharmacovigilance databases established a comprehensive knowledge base of existing treatments, approval timelines, and safety profiles. Industry conference proceedings and expert presentations supplemented this groundwork by illuminating emerging trends and investigational therapies.
Complementing this desk research, structured interviews with key opinion leaders-including hematologists, rare disease specialists, healthcare payers, and patient advocacy representatives-provided invaluable firsthand perspectives on treatment challenges, access barriers, and real-world utilization patterns. These insights were further validated through engagements with leading biopharmaceutical executives and contract research organizations to ensure alignment with corporate pipeline strategies and manufacturing considerations.
To guarantee data integrity, findings were triangulated across multiple sources and subjected to peer review by an independent advisory panel. Quality control measures included consistency checks, bias mitigation protocols, and verification of clinical and regulatory facts. This robust methodology underpins the credibility of the insights presented, offering a transparent and replicable framework for rare disease market analysis.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Gaucher Disease Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Gaucher Disease Treatment Market, by Treatment Type
- Gaucher Disease Treatment Market, by Disease Type
- Gaucher Disease Treatment Market, by Administration Mode
- Gaucher Disease Treatment Market, by End User
- Gaucher Disease Treatment Market, by Patient Group
- Gaucher Disease Treatment Market, by Region
- Gaucher Disease Treatment Market, by Group
- Gaucher Disease Treatment Market, by Country
- United States Gaucher Disease Treatment Market
- China Gaucher Disease Treatment Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1749 ]
Synthesizing Core Insights from Gaucher Disease Treatment Trends to Inform Future Research, Policy Decisions, and Collaborative Opportunities
The evolving Gaucher disease treatment landscape reflects a confluence of scientific breakthroughs, policy adaptations, and patient-centered care innovations. From the expansion of oral substrate reduction agents to the promise of chaperone therapies and gene-editing modalities, stakeholders are collectively reshaping therapeutic paradigms to address unmet needs and enhance quality of life. Regional variations underscore the importance of tailored access strategies, while segmentation insights highlight opportunities for targeted intervention across therapy types, disease manifestations, and care settings.
Competitive dynamics continue to drive portfolio diversification and collaborative alliances, positioning leading companies to capitalize on both incremental and transformative advancements. Yet, the cumulative impact of recent trade policies and the imperative for supply chain robustness present concrete challenges that demand proactive risk management. By leveraging actionable recommendations-ranging from enhanced patient support to value-based contracting-industry leaders can navigate these complexities and sustain momentum in rare disease care.
Ultimately, a coordinated approach that integrates innovation, evidence generation, and stakeholder engagement will be pivotal in translating research advances into tangible patient benefits. This summary underscores the necessity of ongoing collaboration among biopharma, healthcare providers, regulators, and patient communities to realize the full potential of emerging therapies and chart a sustainable path forward.
Secure Exclusive Access to In-Depth Gaucher Disease Treatment Insights by Connecting with Ketan Rohom Associate Director for Tailored Sales Consultation
To explore these comprehensive insights and gain unparalleled clarity on the evolving dynamics of Gaucher disease treatment, reach out directly to Ketan Rohom, Associate Director, Sales & Marketing. He will guide you through a tailored consultation to help you leverage our extensive analysis for strategic decision making and patient-centric program development. Secure your access today to drive competitive advantage and align your organization with the forefront of rare disease innovation.

- How big is the Gaucher Disease Treatment Market?
- What is the Gaucher Disease Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




