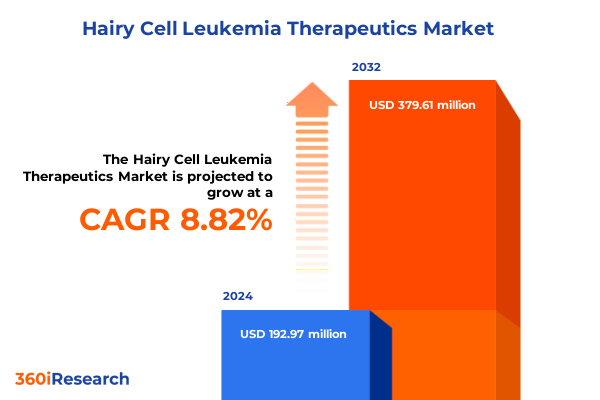
The Hairy Cell Leukemia Therapeutics Market size was estimated at USD 209.04 million in 2025 and expected to reach USD 227.19 million in 2026, at a CAGR of 8.89% to reach USD 379.61 million by 2032.
Exploring the Foundational Biology and Contemporary Treatment Approaches Shaping the Hairy Cell Leukemia Therapeutic Landscape
Hairy cell leukemia is a rare chronic B-cell malignancy characterized by the pathological accumulation of mature clonal lymphocytes in the bone marrow, spleen, and peripheral blood. Its indolent course can mask progressive cytopenias and splenomegaly, often leading to delays in diagnosis until patients present with significant hematologic abnormalities or splenic enlargement. Epidemiologically, this malignancy accounts for approximately 2% to 3% of all leukemias, with an annual incidence of 0.28 to 0.30 cases per 100,000 individuals in North America and Europe. A male predominance is observed, with a male-to-female ratio of up to 2:1, and the median age at presentation typically falls in the late 50s, underscoring a demographic that frequently contends with comorbidities and age-related vulnerabilities upon diagnosis.
At the molecular level, hairy cell leukemia is defined by a near-ubiquitous activating point mutation in the BRAF gene (V600E), which drives the constitutive activation of the RAS–RAF–MEK–ERK signaling cascade. This singular oncogenic event is clonal and persists through both initial diagnosis and subsequent relapses, imprinting a stable genomic signature that has enabled the development of targeted inhibitors. Although variant forms devoid of BRAF mutations can harbor alternative MAP2K1 alterations, the classical V600E mutation underlies the characteristic phenotype and survival advantage of hairy cell clones. This molecular insight has transformed both diagnostic and therapeutic paradigms, facilitating precision-driven treatment strategies that directly engage pathognomonic pathways.
Historically, pentostatin and cladribine-purine nucleoside analogs delivered via intravenous infusion-have formed the backbone of first-line therapy, leveraging their potent cytotoxic effects to induce deep and durable remissions. However, limitations in managing relapsed or refractory disease, coupled with concerns around myelosuppression and long-term toxicity, have galvanized research into monoclonal antibodies, immunotoxins, and small molecule inhibitors. Among these, interferon alfa and rituximab immunotherapy introduced immunomodulatory approaches, while the advent of BRAF and MEK inhibitors has provided oral targeted options. More recently, the CD22-directed cytotoxin moxetumomab pasudotox offered a chemotherapy-free alternative for heavily pretreated patients, although its commercial uptake has been challenged by market dynamics and delivery complexities.
Uncovering the Paradigm-Altering Innovations and Breakthrough Advances Redefining Hairy Cell Leukemia Treatment Scenarios
The therapeutic landscape for hairy cell leukemia has undergone a profound evolution driven by the elucidation of its molecular underpinnings and the translation of those insights into targeted interventions. The identification of BRAF V600E as a disease-defining mutation catalyzed the development of small molecule inhibitors such as vemurafenib and dabrafenib. These agents, delivered orally in tablet form, have demonstrated high rates of rapid hematologic improvement, offering a more tolerable side effect profile than traditional cytotoxic compounds. Concurrently, the recognition that downstream MEK activation contributes to therapeutic resistance spurred the integration of MEK inhibitors like trametinib into combination regimens, further extending durability of response and mitigating escape pathways. This shift to precision-driven therapies has redefined expectations for remission quality and has underscored the value of genomic testing at diagnosis and relapse.
Alongside small molecule innovations, immunotherapeutic strategies have advanced significantly. Monoclonal antibody therapy targeting CD20 with rituximab augmented the activity of purine analogs by selectively depleting B-cell populations, while interferon alfa provided early proof of concept for harnessing immune modulation in HCL. Most notably, moxetumomab pasudotox introduced a novel immunotoxin mechanism by linking a CD22-binding antibody fragment to a Pseudomonas exotoxin payload, producing durable complete remissions in a subset of heavily pretreated patients and spotlighting the potential of toxin-conjugate modalities. Moreover, emerging research into CAR T-cell approaches-such as brexucabtagene autoleucel from Kite Pharma-signals the potential to leverage adoptive cell therapies in the relapsed or refractory setting, further expanding the arsenal of transformative treatment options.
Assessing the Multifaceted Consequences of Proposed U.S. Trade Levies on the Hairy Cell Leukemia Therapeutics Supply Ecosystem
In mid-2025, U.S. policymakers proposed sweeping trade levies on imported pharmaceuticals as part of broader efforts to bolster domestic manufacturing, suggesting duties that could reach double-digit levels across various supply chains. While manufacturers and investors expressed skepticism about the immediacy and scope of enforcement, the specter of substantial future tariffs has already influenced strategic planning. Market participants are closely monitoring the grace period timelines and national security reviews that could convert provisional investigations into binding duties, recognizing that even delayed implementation may exert upward pressure on drug costs and disrupt the timing of new therapy launches.
These potential trade measures carry particular weight for hairy cell leukemia therapeutics, which depend on global supply chains for active pharmaceutical ingredients, specialized bioprocessing components, and advanced delivery systems. With a baseline tariff regime proposed on key imports and the threat of retaliatory measures from major trading partners, industry stakeholders face compounding risks. Manufacturers are evaluating the feasibility and cost of reshoring critical API production, even as they weigh the investments required to retrofit existing facilities. At the same time, distributors and specialty pharmacies are reassessing inventory buffers to mitigate potential shortages, and healthcare providers are scrutinizing budget forecasts to anticipate possible price increases that could impact treatment accessibility and continuity of care.
Illuminating Critical Patient Pathways and Channel Dynamics Driving Segmented Insights Within the Hairy Cell Leukemia Therapeutic Arena
The administration route of hairy cell leukemia therapies exerts a profound influence on patient management, resource allocation, and clinical outcomes. Intravenous infusions remain the paradigm for purine analogs such as cladribine and pentostatin, necessitating inpatient or outpatient infusion facilities and close hematologic monitoring during treatment cycles. By contrast, oral tablet formulations of BRAF and MEK inhibitors offer greater flexibility, enabling patients to adhere to home-based regimens with telemedicine support and digital adherence tools. These divergent pathways underscore the importance of aligning administration route with patient preference, logistical feasibility, and healthcare infrastructure to maximize therapeutic adherence and minimize disruptions in care delivery.
Dosage formulations further differentiate treatment options within the hairy cell leukemia arsenal. Liquid concentrates for infusion allow precise dose adjustments tailored to renal function and body weight, while stand-alone tablets simplify supply chain logistics and patient self-administration. Distribution channels mirror these distinctions: traditional hospital pharmacies manage complex infusion therapies, whereas online specialty pharmacies can dispense oral agents with direct-to-patient shipping, enhancing convenience and reducing travel burdens. In parallel, hospitals and specialty clinics serve as critical endpoints for administering high-intensity regimens and managing adverse events associated with both cytotoxic and immunotoxin treatments. When considering treatment sequencing, first-line approaches leverage purine analogs and immunotherapy in controlled clinical environments, whereas relapsed or refractory lines increasingly incorporate targeted oral agents and novel biotherapeutics, reflecting a maturation of the therapeutic algorithm and an expansion of patient-centric delivery models. Through this multilayered segmentation lens, stakeholders can tailor drug development, commercial strategies, and patient support programs to optimize each modality’s clinical and operational footprint.
This comprehensive research report categorizes the Hairy Cell Leukemia Therapeutics market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Route Of Administration
- Dosage Form
- Treatment Line
- Mechanism Of Action
- Distribution Channel
- End User
Deciphering the Regional Variation in Treatment Adoption and Infrastructure Strength Across Global Zones Influencing Hairy Cell Leukemia Care
The Americas region exhibits robust uptake of the latest targeted therapies and immunotoxins, driven by high per capita healthcare spending, comprehensive reimbursement frameworks, and an advanced network of cancer centers equipped for precision medicine. The United States, in particular, leads in integrating multi-modality regimens and conducting pivotal clinical trials for novel agents, benefiting from well-established pathways for orphan drug designation and expedited review. Leveraging this environment, manufacturers and providers collaborate in real time to optimize dosing strategies, patient education, and post-treatment surveillance, ensuring that innovations translate rapidly into improved clinical outcomes and sustained remission durations across diverse patient populations.
In Europe, Middle East, and Africa, regulatory harmonization under the European Medicines Agency enables centralized evaluation of new treatments, though variable national reimbursement policies and disparities in healthcare infrastructure can modulate market access. Orphan drug incentives have catalyzed submissions for therapies like moxetumomab pasudotox, yet commercial discontinuation decisions and supply constraints have tempered uptake in certain markets. Similarly, emerging economies across the Middle East and Africa are prioritizing capacity building for oncology services, focusing on expanding infusion centers and enhancing diagnostic capabilities to support broader adoption of both legacy and novel therapies. Asia-Pacific markets, marked by low disease prevalence but rapidly evolving healthcare systems, present unique opportunities for licensing partnerships, localized production of generics, and telehealth-enabled follow-up care, as governments invest in rare disease registries and reimbursement reforms to ensure equitable access to breakthrough treatments across the region.
This comprehensive research report examines key regions that drive the evolution of the Hairy Cell Leukemia Therapeutics market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Unveiling Corporate Leadership and Strategic Alliances Shaping the Competitive Hairy Cell Leukemia Therapeutic Landscape
AstraZeneca, as the innovator behind the CD22-directed immunotoxin, has navigated both regulatory milestones and commercial challenges, particularly evident in the strategic withdrawal of moxetumomab pasudotox in key regions despite demonstrating durable responses in relapsed or refractory populations. Meanwhile, Novartis and Roche have positioned their BRAF inhibitors and MEK inhibitors at the forefront of targeted therapy portfolios, investing in combination trials to enhance depth of remission and manage resistance. Pfizer, with its heritage in generic purine analogs and monoclonal antibodies, continues to leverage scale in distribution and volume contracting to maintain competitive pricing and broad formulary access. Each of these corporations has forged alliances-whether through licensing agreements, co-development partnerships, or manufacturing collaborations-to align global supply chains, mitigate tariff risks, and accelerate time to treatment for patients worldwide.
Beyond the primary incumbents, emerging players have enriched the ecosystem through specialized compounds and cell-based therapies. Gilead Sciences, via Kite Pharma, has extended CAR T-cell expertise to B-cell malignancies, while Teva and SuperGen maintain a foothold in purine analogs production, ensuring continuity of first-line options. Johnson & Johnson and Merck KGaA augment this landscape through investments in antibody-drug conjugates and pipeline candidates targeting minimal residual disease. Across the spectrum, these companies balance legacy assets with cutting-edge research, creating a dynamic competitive environment that drives continuous innovation, fosters differentiated value propositions, and ultimately enhances therapeutic choice for clinicians and patients alike.
This comprehensive research report delivers an in-depth overview of the principal market players in the Hairy Cell Leukemia Therapeutics market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Amgen Inc.
- Apollo Scientific
- AstraZeneca PLC
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Biogenomics Limited
- Bristol-Myers Squibb Company
- Cilag AG
- Clinigen Limited
- Daiichi Sankyo Company, Limited
- Dr. Reddy's Laboratories Ltd.
- F. Hoffmann-La Roche AG
- Fresenius SE and Co. KGaA
- Johnson & Johnson Services, Inc.
- Lipomed AG
- Manus Aktteva Biopharma LLP
- Merck KGaA
- Midas Pharma GmbH
- Novartis AG
- Pfizer Inc.
- Qiagen NV
- Sumitomo Dainippon Pharma Co., Ltd
- Veol Medical Technologies Pvt Ltd.
- Zhejiang Hisun Pharmaceutical Co., Ltd.
Driving Proactive Strategies and Collaborative Frameworks to Enhance Patient Access and Innovation in Hairy Cell Leukemia Therapeutics
To navigate the emerging tariff landscape and fortify supply chain resilience, industry leaders should prioritize dual sourcing strategies for critical active pharmaceutical ingredients, balancing domestic production capabilities with international partnerships. This approach will mitigate the risk of sudden cost escalations and supply interruptions, while fostering long-term stability in manufacturing pipelines. Concurrently, investing in continuous process verification and advanced analytics will streamline regulatory compliance and reduce lead times, ensuring that new and existing therapies remain accessible amid shifting trade policies. Collaboration with governmental agencies to refine harmonized standards for API quality and traceability can further fortify these efforts, aligning commercial interests with public health imperatives.
In parallel, expanding patient support programs to encompass digital health platforms and telemedicine services will enhance adherence and care continuity, particularly for those receiving oral targeted therapies at home. By integrating remote monitoring tools, patient-reported outcomes, and virtual education modules, companies can personalize treatment pathways and preemptively address adverse events. This digital engagement model not only elevates patient experience but also generates real-world evidence to refine clinical protocols and inform value-based contracting. Establishing cross-stakeholder forums that include payers, providers, and patient advocacy groups will expedite consensus on outcome measures, reimbursement criteria, and access programs, driving a unified approach to delivering transformative therapies to hairy cell leukemia patients.
From an R&D perspective, forging public-private partnerships for next-generation modalities-such as bispecific antibodies, CAR T cells, and novel immunotoxin constructs-will distribute risk, leverage pooled expertise, and accelerate clinical development. Aligning trial designs with adaptive protocols and biomarker-driven endpoints can enhance the translation of early signals into definitive clinical benefit, supporting more efficient regulatory pathways. Concurrently, expanding genomic sequencing initiatives and minimal residual disease tracking will inform treatment sequencing and refine patient stratification, maximizing therapeutic impact and minimizing unnecessary toxicity. By championing these collaborative and data-driven strategies, industry leaders can mitigate external pressures, elevate the standard of care, and sustain momentum in the fight against this rare hematologic malignancy.
Detailing the Rigorous Multisource Analytical Approach Underpinning the Hairy Cell Leukemia Therapeutic Research Study
This analysis was underpinned by a multi-phased research methodology that integrated comprehensive secondary data aggregation, stakeholder interviews, and rigorous qualitative validation. Secondary data encompassed peer-reviewed publications, regulatory databases from the FDA and EMA, corporate financial reports, and published clinical trial results. Publicly accessible repositories such as the National Cancer Institute archives and the European Public Assessment Reports provided critical insights into approval histories and designation statuses. To ensure robust triangulation, this foundation was augmented by targeted discussions with hematology-oncology experts, pharmacoeconomists, and manufacturing specialists, enabling real-time calibration of market trends and policy impacts.
Primary research included in-depth interviews with key opinion leaders across oncology centers, specialty clinics, and distribution networks, along with surveys of patient advocacy organizations to capture real-world access challenges. Data synthesis employed thematic analysis to distill actionable insights from qualitative narratives, while quantitative inputs were normalized using consistent nomenclature and cross-verified against multiple sources to mitigate bias. Throughout the process, findings were subjected to peer review by an internal advisory panel comprising regulatory affairs, clinical development, and supply chain experts, ensuring that conclusions reflect both strategic rigor and operational pragmatism.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Hairy Cell Leukemia Therapeutics market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Hairy Cell Leukemia Therapeutics Market, by Route Of Administration
- Hairy Cell Leukemia Therapeutics Market, by Dosage Form
- Hairy Cell Leukemia Therapeutics Market, by Treatment Line
- Hairy Cell Leukemia Therapeutics Market, by Mechanism Of Action
- Hairy Cell Leukemia Therapeutics Market, by Distribution Channel
- Hairy Cell Leukemia Therapeutics Market, by End User
- Hairy Cell Leukemia Therapeutics Market, by Region
- Hairy Cell Leukemia Therapeutics Market, by Group
- Hairy Cell Leukemia Therapeutics Market, by Country
- United States Hairy Cell Leukemia Therapeutics Market
- China Hairy Cell Leukemia Therapeutics Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1272 ]
Synthesizing Core Insights to Propel Strategic Decision Making and Future Directions in Hairy Cell Leukemia Therapeutic Development
In synthesizing these findings, it is evident that the hairy cell leukemia therapeutic landscape is defined by a convergence of molecular precision, immunologic innovation, and operational agility. The maturation of targeted therapies and immunotoxins has reshaped remission benchmarks, while forthcoming modalities such as CAR T-cell constructs promise to further disrupt conventional paradigms. However, external pressures-ranging from proposed trade levies to regulatory and reimbursement variability-underscore the imperative for adaptive strategies that safeguard patient access and sustain innovation pipelines.
Looking ahead, stakeholders must maintain a forward-leaning posture, integrating real-world evidence frameworks, public-private partnerships, and digital health enablers to navigate an increasingly complex environment. By balancing risk-mitigation in the supply chain with dynamic clinical development models, the industry can ensure that progress against this rare disease translates into tangible benefits for patients. Ultimately, the depth of collaboration between manufacturers, payers, providers, and advocacy organizations will determine the success of delivering next-generation therapies and securing durable outcomes for individuals living with hairy cell leukemia.
Connect Directly With the Associate Director of Sales & Marketing to Secure Comprehensive Data on Hairy Cell Leukemia Therapeutics Insights
To explore how your organization can leverage the comprehensive insights and strategic frameworks detailed in this report, reach out to Ketan Rohom, Associate Director of Sales & Marketing, who is prepared to guide you through tailored offerings and secure access to the full analysis and data that will empower your decision-making processes.

- How big is the Hairy Cell Leukemia Therapeutics Market?
- What is the Hairy Cell Leukemia Therapeutics Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




