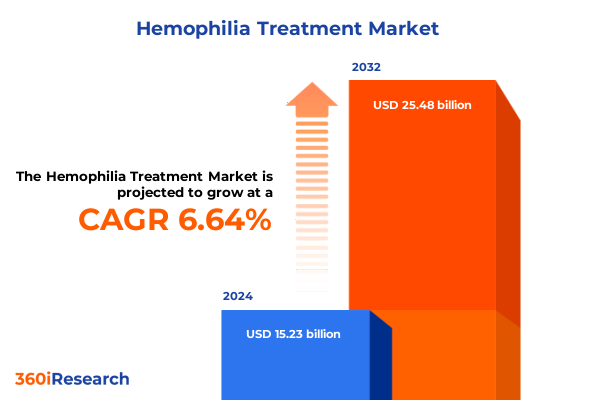
The Hemophilia Treatment Market size was estimated at USD 16.26 billion in 2025 and expected to reach USD 17.24 billion in 2026, at a CAGR of 6.62% to reach USD 25.48 billion by 2032.
Exploring the Evolution and Current State of Hemophilia Treatment to Uncover Strategic Opportunities and Emerging Challenges
Hemophilia, a rare genetic bleeding disorder, has challenged clinicians and patients for decades due to its lifelong risk of uncontrolled hemorrhage and joint damage. Historically, treatment revolved around frequent infusions of clotting factor replacement therapies, demanding rigorous adherence and presenting logistical burdens for patients and caregivers alike. The pursuit of more durable and less invasive solutions has driven relentless innovation across the hemophilia treatment ecosystem.
In recent years, gene therapies have emerged as transformative options, offering the potential for sustained factor expression following a single infusion. Concurrently, extended half-life replacement products and novel antithrombin-lowering agents have entered clinical practice, reshaping prophylactic strategies and reducing treatment frequency. These therapeutic advances have redefined patient expectations and introduced new considerations around safety, durability, and cost management.
As the landscape evolves, stakeholders must navigate regulatory dynamics, supply chain complexities, and shifting payer frameworks. This executive summary synthesizes critical developments across therapeutic classes, regional markets, tariff impacts, and competitive positioning. Through comprehensive insights, it equips decision-makers with the context necessary to chart a course toward sustainable growth and improved patient outcomes.
Groundbreaking Innovations in Gene Editing, Extended Half-Life Products, and Personalized Prophylaxis Reshaping Hemophilia Treatment Landscapes
The hemophilia treatment landscape has witnessed an unprecedented wave of innovation, driven by advances in gene editing, viral vector design, and biologic engineering. Leading gene therapies, such as etranacogene dezaparvovec-drlb, have demonstrated sustained factor IX activity and near-normal clotting performance four years post-infusion without continuous prophylaxis in adults with hemophilia B. This durability marks a paradigm shift away from lifelong infusion regimens toward potentially one-time interventions with durable clinical benefits.
Simultaneously, extended half-life clotting factor products and RNA-based therapies have gained traction, offering tailored prophylactic options that reduce infusion frequency and improve patient quality of life. For instance, bispecific antibodies and antithrombin-lowering small-interfering RNAs have shown over 90% reductions in annualized bleed rates in both hemophilia A and B cohorts. These modalities are driving personalized prophylaxis regimens, leveraging patient-specific bleeding risk profiles to optimize dosing intervals.
Digital health integration, encompassing remote monitoring and mobile adherence platforms, is reinforcing these therapeutic advances. By enabling real-time bleed tracking and personalized alerts, digital tools facilitate proactive management and timely clinical interventions. Together, these transformative shifts are setting new standards in efficacy, safety, and patient-centric care, requiring stakeholders to recalibrate development pipelines and commercialization strategies.
Analyzing the Aggregate Consequences of Emerging 2025 U.S. Trade Tariffs on Hemophilia Treatment Supply Chains and Cost Structures
In April 2025, the U.S. government enacted a comprehensive 10% tariff on nearly all imported healthcare goods, encompassing active pharmaceutical ingredients, biologics, and medical devices. This sweeping approach aims to rebuild domestic manufacturing capacity but has introduced immediate cost pressures for pharmaceutical manufacturers and healthcare providers alike. For hemophilia treatment, which relies on complex biologics and specialized equipment, these additional duties threaten to elevate manufacturing and distribution expenses.
More aggressive country-specific measures have further strained the supply chain. Tariffs of up to 245% on Chinese APIs, alongside 25% duties on medical products from Canada and Mexico for non-compliant USMCA imports, have forced companies to reassess sourcing strategies and consider nearshoring. The resulting cost increases have prompted delays in manufacturing expansions, supply bottlenecks for replacement therapies, and potential price escalations for end users, undermining patient access and payer budgets.
Regulatory uncertainty compounds these challenges. Legal injunctions, such as the V.O.S. Selections decision halting ‘‘Liberation Day’’ tariffs under IEEPA authority, underscore the fragility of executive-driven trade actions. Concurrently, initiatives like the Most-Favored-Nation Drug Pricing Executive Order introduce alternative cost-containment pressures by tying U.S. prices to lower international benchmarks. Together, these cumulative factors demand strategic agility and robust risk mitigation from industry leaders.
Deep Dive into Hemophilia Treatment Segmentation Reveals Critical Nuances Across Therapeutic Classes, Delivery Modes, Care Settings, and Supply Channels
A nuanced understanding of therapeutic classes reveals the divergent growth trajectories of gene therapy and replacement therapy. Gene therapy’s one-time infusion model aims to deliver durable factor expression, reshaping how clinicians approach long-term bleed prophylaxis. In comparison, replacement therapies continue to evolve with extended half-life formulations that reduce administration frequency and enhance patient adherence through subcutaneous and low-volume delivery systems.
The balance between on-demand and prophylactic treatment modes underscores varying patient needs and healthcare strategies. On-demand interventions remain critical for acute bleed management, yet the shift toward routine prophylaxis has gained momentum. Personalized prophylaxis regimens integrate bleed history, lifestyle factors, and emerging biomarkers to tailor dosing intervals, improving both patient outcomes and resource utilization within care networks.
Diverse care environments, from home healthcare settings to specialized clinics and hospital-based infusion centers, have unique infrastructure and reimbursement models. Home-based infusions empower patients with self-administration flexibility but hinge on robust patient education and support. Meanwhile, hospital pharmacies and retail outlets shape distribution channels, influencing therapy accessibility and cost negotiations. Understanding these interrelated segmentation dynamics is vital for optimizing market entry and distribution strategies.
This comprehensive research report categorizes the Hemophilia Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Therapeutic Class
- Treatment Mode
- End User
- Distribution Channel
Uncovering Regional Divergences in Hemophilia Treatment Adoption, Regulatory Frameworks, and Market Dynamics Across Key Global Geographies
The Americas remain at the forefront of hemophilia treatment innovation, anchored by the United States’ advanced regulatory framework and reimbursement environment. The FDA’s accelerated pathways have facilitated gene therapy approvals, while payer collaborations and outcome-based contracting models are enabling broader patient access. Canada’s provincial healthcare systems, though more centralized, are adapting innovative payment models to accommodate high-value therapies and support equitable distribution.
Across Europe, the Middle East, and Africa, diverse regulatory landscapes and pricing controls influence market dynamics. Established healthcare systems in Western Europe have adopted value-based assessments to balance cost and therapeutic benefit, while emerging economies in the Middle East and North Africa face challenges around budget constraints and supply chain reliability. The European Union’s centralized approval mechanisms streamline access, yet national reimbursement decisions vary significantly by country.
In Asia-Pacific, rapid healthcare infrastructure expansion and growing patient advocacy are driving momentum for novel therapies. Japan’s robust medical reimbursement environment supports expedited gene therapy reviews, while China’s recent inclusion of biologics in national insurance schemes signals an expanding market. Nevertheless, complex import regulations and local manufacturing requirements can delay entry, requiring tailored regional strategies to navigate evolving regulatory and reimbursement landscapes.
This comprehensive research report examines key regions that drive the evolution of the Hemophilia Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Insight into Major Hemophilia Treatment Players Highlighting Diverse Strategic Portfolios Spanning Gene Therapy, Replacement, and Novel Prophylactic Agents
Leading biopharmaceutical organizations have strategically diversified their hemophilia portfolios to capture evolving market needs. CSL’s etranacogene dezaparvovec-drlb has set a benchmark for long-term factor IX restoration, demonstrating over 90% reduction in bleeding rates and sustained factor activity through four years post-infusion. This gene therapy positions CSL as a pioneer in one-time treatments, challenging traditional replacement paradigms.
Sanofi’s Qfitlia leverages an innovative antithrombin-lowering mechanism to deliver consistent prophylaxis with six annual injections, reshaping patient convenience and adherence. Its broad label covering hemophilia A and B with or without inhibitors further extends its competitive advantage, supported by specialized companion diagnostic tools that streamline patient selection and monitoring.
Pfizer continues to reinforce its late-stage pipeline, with Hympavzi achieving a 93% reduction in annualized bleed rates for patients with specific antibodies , and its forthcoming hemophilia A gene therapy demonstrating superior bleed control to standard factor VIII replacement. Additionally, BioMarin’s Roctavian has maintained durable factor VIII activity and multi-year bleed protection, illustrating the commercial viability of gene therapies in hemophilia A. These strategic moves highlight the competitive interplay between established biologics and next-generation cures.
This comprehensive research report delivers an in-depth overview of the principal market players in the Hemophilia Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Alnylam Pharmaceuticals, Inc.
- Bayer AG
- BioMarin Pharmaceutical Inc.
- Centessa Pharmaceuticals plc
- CSL Behring LLC
- Expression Therapeutics LLC
- F. Hoffmann-La Roche Ltd
- Freeline Therapeutics Holdings plc
- GC Biopharma Corp.
- Grifols, S.A.
- Kedrion Biopharma Inc.
- Novo Nordisk A/S
- Octapharma AG
- Pfizer Inc.
- Sangamo Therapeutics, Inc.
- Sanofi S.A.
- Spark Therapeutics, Inc.
- Swedish Orphan Biovitrum AB (publ)
- Takeda Pharmaceutical Company Limited
- uniQure N.V.
Strategic Measures Industry Leaders Must Implement to Navigate Complex Tariff Regimes, Accelerate Innovation, and Enhance Patient-Centered Hemophilia Care
To navigate escalating tariff pressures and regulatory uncertainties, leaders should establish resilient supply chain frameworks that incorporate regional manufacturing hubs and diversified API sourcing. By forging strategic alliances with contract manufacturing organizations domestically and abroad, companies can mitigate duty exposure and ensure uninterrupted access to critical biologics. Concurrently, investing in process optimization and digital track-and-trace solutions will reinforce compliance and cost efficiency.
Innovation pipelines must align with shifting payer expectations and value-based care models. Prioritizing therapies with demonstrable durability and patient-reported outcome benefits can unlock premium reimbursement pathways. Furthermore, integrating companion diagnostics and real-world evidence platforms will strengthen value propositions and facilitate outcome-driven contracting. Engaging early with payers and regulatory bodies to define evidence requirements will accelerate time to reimbursement and market access.
Cultivating patient-centric ecosystems through digital health initiatives is equally crucial. Remote monitoring, tele-infusion support, and mobile adherence platforms not only enhance compliance but generate real-time data for personalized treatment adjustments. By partnering with patient advocacy groups and healthcare providers, industry leaders can co-create comprehensive support programs that improve quality of life and foster long-term engagement.
Comprehensive Research Methodology Integrating Robust Primary Engagement and Rigorous Secondary Analysis to Ensure Data Reliability and Actionable Insights
This research integrates a multi-tiered approach combining robust secondary data analysis and strategic primary engagements. Key information sources include regulatory filings from the FDA and EMA, trade policy documentation, and peer-reviewed clinical outcomes. Market dynamics were evaluated through a synthesis of industry reports, public company disclosures, and global trade tribunal decisions to ensure comprehensive context around tariff impacts and legal precedents.
Qualitative insights were garnered via in-depth interviews with leading hematologists, supply chain experts, and market access professionals. These stakeholders provided nuanced perspectives on therapy adoption, reimbursement challenges, and patient journey considerations. Data triangulation techniques were employed to validate findings across sources, ensuring methodological rigor and minimizing bias in scenario interpretations.
Segmentation analyses were meticulously structured to reflect therapeutic class, treatment mode, end users, and distribution channels. Regional assessments incorporated country-level policy reviews and healthcare system classifications. Competitive intelligence was derived from clinical trial registries, partnership announcements, and patent landscape evaluations. The resulting framework delivers actionable intelligence, underpinned by transparent documentation of sources, assumptions, and analytical processes.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Hemophilia Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Hemophilia Treatment Market, by Therapeutic Class
- Hemophilia Treatment Market, by Treatment Mode
- Hemophilia Treatment Market, by End User
- Hemophilia Treatment Market, by Distribution Channel
- Hemophilia Treatment Market, by Region
- Hemophilia Treatment Market, by Group
- Hemophilia Treatment Market, by Country
- United States Hemophilia Treatment Market
- China Hemophilia Treatment Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 795 ]
Conclusion Synthesizes Key Market Developments, Regulatory Influences, and Strategic Imperatives Shaping the Future of Hemophilia Treatment
The hemophilia treatment landscape is undergoing a profound transformation driven by gene therapies, novel prophylactic modalities, and evolving digital care models. These advances are redefining the balance between efficacy, safety, and patient convenience, while introducing new strategic imperatives around pricing, access, and supply chain resilience. Stakeholders must embrace holistic approaches that integrate clinical innovation with adaptive market strategies.
Tariff-induced cost pressures and regulatory volatility underscore the importance of diversified manufacturing footprints and proactive policy engagement. Legal developments, such as court challenges to emergency tariffs and the introduction of most-favored-nation pricing frameworks, highlight the dynamic intersection of trade policy and healthcare economics. Navigating these complexities will require cross-functional collaboration and agile governance structures.
As competition intensifies, differentiation will hinge on value propositions that encompass clinical outcomes, economic benefits, and patient-centric services. By aligning pipeline priorities with payer evidence requirements and leveraging real-world data, industry leaders can secure sustainable market positions. The insights presented herein offer a strategic roadmap for capitalizing on emerging opportunities and mitigating risks in the rapidly evolving hemophilia treatment domain.
Take the Next Step to Empower Your Hemophilia Strategy Today by Accessing the Full Market Research Report through Direct Engagement with Our Sales Leadership
By securing the full market research report, decision-makers can gain unparalleled visibility into treatment innovations, tariff implications, and competitive strategies that define the hemophilia landscape. Engaging directly with our team ensures tailored guidance, from granular segmentation analyses to regional opportunity mapping, all designed to amplify your strategic positioning.
To explore how these insights can drive growth and resilience in your organization, reach out to Ketan Rohom, Associate Director, Sales & Marketing. His expertise in translating complex data into actionable plans will help you align your investments with emerging opportunities and regulatory realities.
Contacting Ketan Rohom today will unlock immediate access to in-depth findings, customized consulting options, and special package offerings. Elevate your hemophilia treatment strategy now by partnering with our sales leadership to acquire the definitive market intelligence you need.

- How big is the Hemophilia Treatment Market?
- What is the Hemophilia Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




